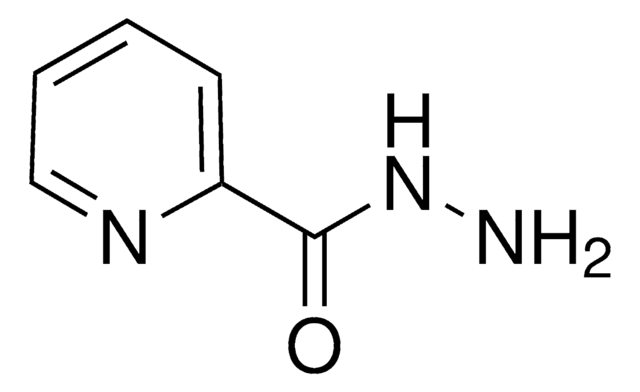
推荐产品
品質等級
化驗
97%
mp
159-161 °C (lit.)
溶解度
H2O: soluble 50 mg/mL
官能基
amine
hydrazine
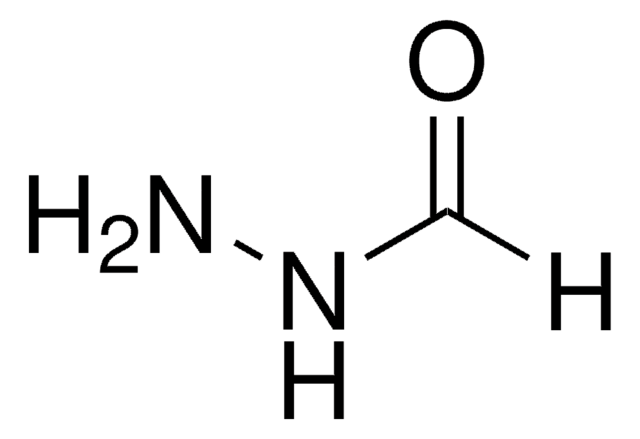
SMILES 字串
NNC(=O)c1cccnc1
InChI
1S/C6H7N3O/c7-9-6(10)5-2-1-3-8-4-5/h1-4H,7H2,(H,9,10)
InChI 密鑰
KFUSANSHCADHNJ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
异烟碱酰肼是一种杂环化合物,可用于合成席夫碱。
應用
烟酰肼用于腙文库形成 。用于研究辣根过氧化物酶对异烟酸酰肼(异烟肼)的氧化作用 。
生化/生理作用
烟酰肼是过氧化物酶的抑制剂 。形成具有较强生物活性的固体金属配合物 。
準備報告
烟酰肼溶于水中,浓度为 50 mg/mL,形成澄清、无色溶液。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
V Goral et al.
Proceedings of the National Academy of Sciences of the United States of America, 98(4), 1347-1352 (2001-02-15)
Dynamic combinatorial libraries are mixtures of compounds that exist in a dynamic equilibrium and can be driven to compositional self adaptation via selective binding of a specific assembly of certain components to a molecular target. We present here an extension
Thermo-chemical behavior of solid nicotinic hydrazide metal complexes in correlation with their stoichiometry.
Sekkina MM and El-Azm MG.
Thermochimica Acta, 77(1), 211-218 (1984)
H A Shoeb et al.
Antimicrobial agents and chemotherapy, 27(3), 399-403 (1985-03-01)
Oxidation of isonicotinic acid hydrazide (isoniazid) by horseradish peroxidase at the expense of H2O2 yielded reactive species which were able to reduce nitroblue tetrazolium and bleach p-nitrosodimethylaniline. Nicotinic acid hydrazide oxidation did not cause these effects. At slightly alkaline pH
Alireza Moradi et al.
Archiv der Pharmazie, 343(9), 509-518 (2010-09-02)
A series of 2-phenoxynicotinic acid hydrazides were synthesized and evaluated for their analgesic and anti-inflammatory activities. Several compounds having an unsubstituted phenyl/4-pyridyl or C-4 methoxy substituent on the terminal phenyl ring showed moderate to high analgesic or anti-inflammatory activity in
[Tautomerism of 2-hydrazino-4-phenylthiazole<-->4-phenylthiazol-2-one hydrazone. Derivatives of acids. II. (4-phenyl-3-R-thiazol-2-ylidene) and beta-methyl-beta-(4-phenylthiazol-2-yl) hydrazides of picolinic, nicotinic, and isonicotinic acid].
L Bielak et al.
Annales Universitatis Mariae Curie-Sklodowska. Sectio D: Medicina, 44, 41-51 (1989-01-01)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门