T6513
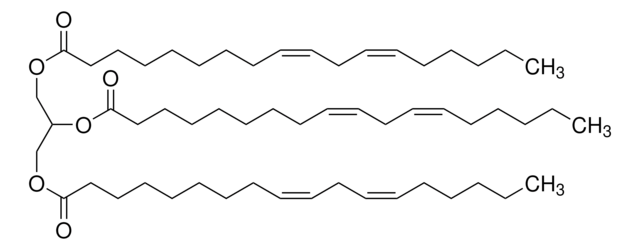
Glyceryl trilinolenate
≥97% (TLC), liquid
Synonym(s):
TG(18:3(9Z,12Z,15Z)/18:3(9Z,12Z,15Z)/18:3(9Z,12Z,15Z)), 1,2,3-Tri-(cis,cis,cis-9,12,15-octadecatrienoyl)glycerol, 1,2,3-Trilinolenoylglycerol, Glycerol trilinolenate, Trilinolenin
About This Item
Recommended Products
biological source
Linum usitatissimum oil
Quality Level
Assay
≥97% (TLC)
form
liquid
refractive index
n20/D 1.489
density
0.94 g/mL at 20 °C (lit.)
functional group
ester
lipid type
neutral glycerides
shipped in
dry ice
storage temp.
−20°C
SMILES string
CC\C=C/C\C=C/C\C=C/CCCCCCCC(=O)OCC(COC(=O)CCCCCCC\C=C/C\C=C/C\C=C/CC)OC(=O)CCCCCCC\C=C/C\C=C/C\C=C/CC
InChI
1S/C57H92O6/c1-4-7-10-13-16-19-22-25-28-31-34-37-40-43-46-49-55(58)61-52-54(63-57(60)51-48-45-42-39-36-33-30-27-24-21-18-15-12-9-6-3)53-62-56(59)50-47-44-41-38-35-32-29-26-23-20-17-14-11-8-5-2/h7-12,16-21,25-30,54H,4-6,13-15,22-24,31-53H2,1-3H3/b10-7-,11-8-,12-9-,19-16-,20-17-,21-18-,28-25-,29-26-,30-27-
InChI key
UBEIMDKGOYBUKT-FLIQGJDUSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Quantitation of furan and methylfuran formed in different precursor systems by proton transfer reaction mass spectrometry.: The research quantifies furan and methylfuran production from various precursors using advanced mass spectrometry techniques. These insights are important for food safety and understanding the chemical reactions during food processing (Märk et al., 2006).
Packaging
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service