SML0847
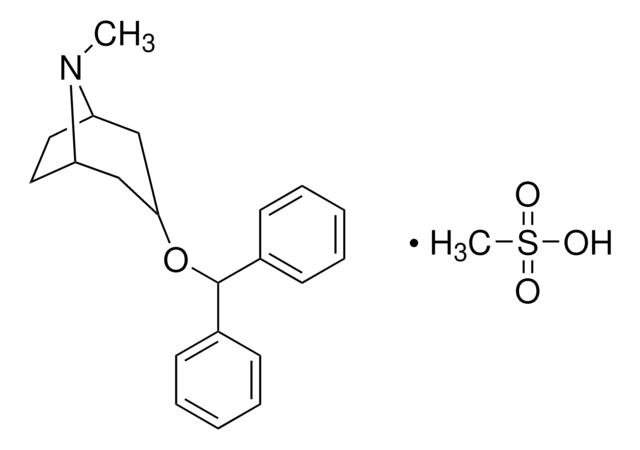
Benztropine mesylate
≥98% (HPLC)
Synonym(s):
Benzatropine mesylate, Benzotropine mesylate, Benztropine methanesulfonate
About This Item
Recommended Products
Assay
≥98% (HPLC)
form
powder
storage condition
desiccated
color
white to beige
solubility
H2O: 20 mg/mL, clear
storage temp.
2-8°C
SMILES string
CS(O)(=O)=O.CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(c3ccccc3)c4ccccc4
InChI
1S/C21H25NO.CH4O3S/c1-22-18-12-13-19(22)15-20(14-18)23-21(16-8-4-2-5-9-16)17-10-6-3-7-11-17;1-5(2,3)4/h2-11,18-21H,12-15H2,1H3;1H3,(H,2,3,4)/t18-,19+,20+;
InChI key
CPFJLLXFNPCTDW-BWSPSPBFSA-N
Gene Information
human ... CHRM1(1128)
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
These distinct transporters, NET, DAT and SERT, respectively, are of particular clinical interest because they are the molecular targets for many antidepressants as well as drugs of abuse, such as cocaine and the amphetamines.
Muscarinic acetylcholine receptors are G protein-coupled receptors (GPCRs) and mediate acetylcholine actions in the CNS and non-nervous tissues. Learn more about acetylcholine receptors and their role in cell signaling.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service