L3295
Phospholipase A1 from Aspergillus oryzae
Synonym(s):
Lecitase™ Ultra, PLA1
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in Aspergillus oryzae
Quality Level
form
liquid
specific activity
≥10 KLU/g
storage temp.
2-8°C
General description
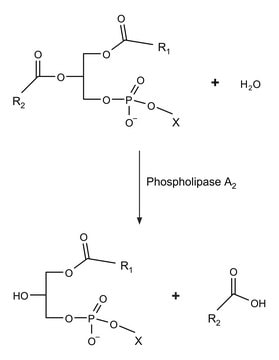
Phospholipase A1 (PLA1) catalyzes the hydrolysis of acyl group from position 1 of lecithin to yield lysolecithin. It is expressed in a wide range of organisms such as rat platelets, bovine brain and testis, hornet venom, bonito muscle and fungi. Gene coding for PLA1 consists of four exons and three short introns spanning 1,056bp of genomic DNA. Mature protein contains 269 aminoacids and two possible N-glycosylation sites (Asn27 and Asn55).
Application
Phospholipase A1 from Aspergillus oryzae has been used:
- in the preparation of sn-1 and sn-2 C18:1- lysophosphatidylcholine (LPC) regioisomer standards
- as a catalyst for the synthesis 6-O-glucosyl-poly(3-hydroxyalkanoates) in a micro-aqueous system
- to catalyze the synthesis of methyl butanoate and methyl benzoate flavor esters in continuous flow microreactor
- to hydrolyze 17:0 phosphocholine (PC)
Analysis Note
minimum activity 10 KLU/G liquid
Legal Information
Lecitase is a trademark of Novozymes Corp.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Thermomyces lanuginosus lipase-catalyzed synthesis of natural flavor esters in a continuous flow microreactor
Gumel AM and Annuar MSM
3 Biotech, 6(1), 24-24 (2016)
Naoaki Arima et al.
Journal of lipid research, 53(3), 513-521 (2011-12-17)
Members of the pancreatic lipase family exhibit both lipase activity toward triacylglycerol and/or phospholipase A(1) (PLA(1)) activity toward certain phospholipids. Some members of the pancreatic lipase family exhibit lysophospholipase activity in addition to their lipase and PLA(1) activities. Two such
Yukichi Fujikawa et al.
Lipids, 47(3), 303-312 (2011-11-30)
A cDNA encoding protein with homology to plant secretory phospholipase A₂ (sPLA₂), denoted as Nt1 PLA₂, was isolated from tobacco (Nicotiana tabacum). The cDNA encodes a mature protein of 118 amino acid residues with a putative signal peptide of 29
Nicole A Housley et al.
Journal of bacteriology, 193(18), 4634-4642 (2011-07-19)
Here we have characterized the Rickettsia prowazekii RP534 protein, a homologue of the Pseudomonas aeruginosa ExoU phospholipase A (PLA) secreted cytotoxin. Our studies showed that purified recombinant RP534 PLA possessed the predicted PLA(2) and lyso-PLA(2) activities based on what has
Alyssa L Bolen et al.
Journal of lipid research, 52(5), 958-970 (2011-03-12)
Platelet activation initiates an upsurge in polyunsaturated (18:2 and 20:4) lysophosphatidic acid (LPA) production. The biochemical pathway(s) responsible for LPA production during blood clotting are not yet fully understood. Here we describe the purification of a phospholipase A(1) (PLA(1)) from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service