H5915
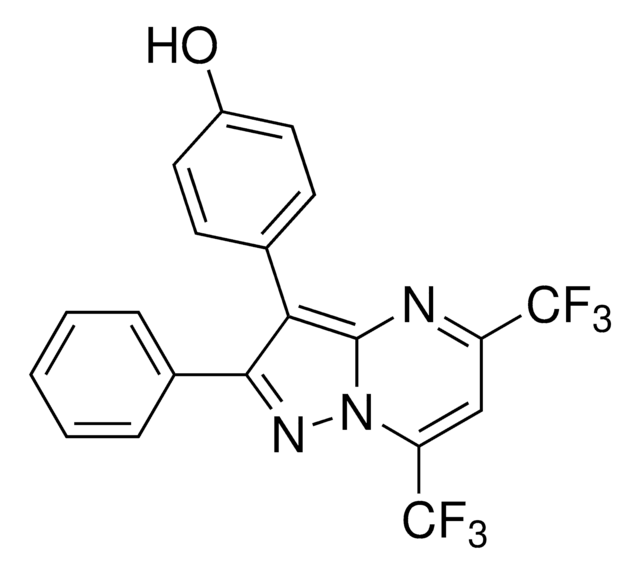
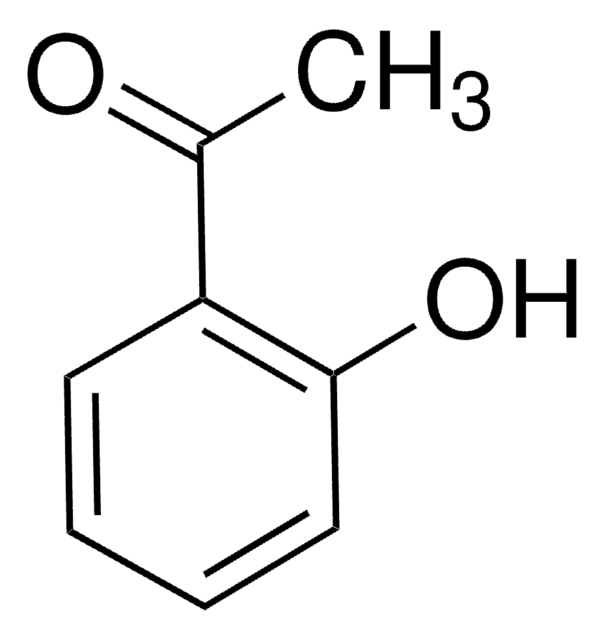
2,3-Bis(4-hydroxyphenyl)propionitrile
≥98% (HPLC)
Synonym(s):
DPN, Diarylpropionitrile, SC-4473
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H13NO2
CAS Number:
Molecular Weight:
239.27
MDL number:
UNSPSC Code:
51111800
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
sterility
non-sterile
Quality Level
Assay
≥98% (HPLC)
form
powder
storage condition
desiccated
color
white to beige
solubility
DMSO: 10 mg/mL, clear
shipped in
ambient
storage temp.
−20°C
SMILES string
Oc1ccc(CC(C#N)c2ccc(O)cc2)cc1
InChI
1S/C15H13NO2/c16-10-13(12-3-7-15(18)8-4-12)9-11-1-5-14(17)6-2-11/h1-8,13,17-18H,9H2
InChI key
GHZHWDWADLAOIQ-UHFFFAOYSA-N
Biochem/physiol Actions
2,3-Bis(4-hydroxyphenyl)-propionitrile (Diarylprepionitrile, DPN) is an ERβ-selective agonist; IC50 = 15nM. DPN protects WT and ARKO mice and significantly decreases IL-1β following LPS treatment in young adult-derived microglia. PPT (Cat. No.H6036, ERa agonist) enhances cell proliferation, while DPN inhibits it. PPT increases Bcl-2 expression, while DPN decreases it. DPN also elevates Bax expression. DPN induces a dose-dependent increase on vitellogenin synthesis. PPT and DPN are effective in dynamically, but differentially regulating intracellular calcium signaling in hippocampal neurons. DPN is more efficacious than PPT in potentiating a physiological concentration of glutamate-induced intracellular Ca2+ rise in these neurons. DPN prevents the development of prostatic hyperplasia and inflammation in testosterone-treated LuRKO mice.
Features and Benefits
This compound is featured on the Nuclear Receptors (Steroids) page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Thomas J Lechuga et al.
Biology of reproduction, 100(2), 514-522 (2018-10-03)
Endogenous hydrogen sulfide (H2S) is a potent vasodilator and proangiogenic second messenger synthesized from L-cysteine by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CTH). Estrogens are potent vasodilators that stimulate H2S biosynthesis in uterine arteries (UA) in vivo; however, the underlying
Chew Leng Lim et al.
eLife, 9 (2020-07-25)
There is strong evidence that the pro-inflammatory microenvironment during post-partum mammary involution promotes parity-associated breast cancer. Estrogen exposure during mammary involution drives tumor growth through neutrophils' activity. However, how estrogen and neutrophils influence mammary involution are unknown. Combined analysis of
L Hases et al.
Scientific reports, 10(1), 16160-16160 (2020-10-02)
There is a strong association between obesity and colorectal cancer (CRC), especially in men, whereas estrogen protects against both the metabolic syndrome and CRC. Colon is the first organ to respond to high-fat diet (HFD), and estrogen receptor beta (ERβ)
Konstantin Yakimchuk et al.
Endocrine connections, 7(12), 1472-1479 (2018-11-30)
Well-defined physiological functions of estrogens are mediated via nuclear estrogen receptors α (ESR1) and β (ESR2). With regard to hematological malignancies, expression of ESR2 has been found in both B and T cell lymphomas. In addition to endogenous estrogens or
Peiye Song et al.
Journal of experimental & clinical cancer research : CR, 38(1), 354-354 (2019-08-16)
Estrogen receptor β (ERβ) has been reported to play an anti-cancer role in breast cancer, but the regulatory mechanism by which ERβ exerts this effect is not clear. Claudin-6 (CLDN6), a tight junction protein, acts as a tumor suppressor gene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service