G3416
5′-Guanidinonaltrindole di(trifluoroacetate) salt hydrate
solid, ≥98% (HPLC)
Synonym(s):
GNTI
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
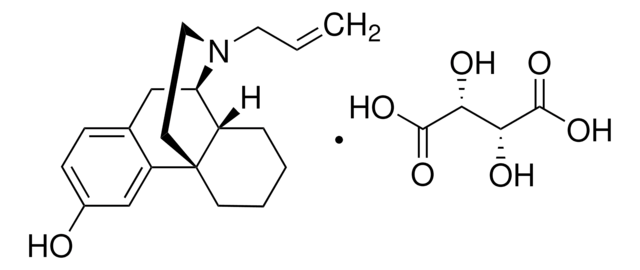
C27H29N5O3 · 2C2HF3O2 · xH2O
CAS Number:
Molecular Weight:
699.60 (anhydrous basis)
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
solid
solubility
H2O: 30 mg/mL
storage temp.
2-8°C
Gene Information
human ... OPRK1(4986)
Application
5′-Guanidinonaltrindole di(trifluoroacetate) salt hydrate (GNTI) has been used as a selective κ opioid receptor antagonist:
- to study the influence of opioid receptor types on the anti-hyperalgesic effect of dipeptidyl peptidase 4 (DPP4) inhibitors in inflammation
- to study the role of κ opioid receptor in the forebrain-dependent associative task, Whisker-Trace Eyeblink conditioning (WTEB)
- to validate its inhibitory actions on Akt kinase activities
Biochem/physiol Actions
5′-Guanidinonaltrindole di(trifluoroacetate) (GNTI) is a 5‘-guanidine derivative and a selective κ opiate receptor antagonist. It is five-fold more potent and 500-fold more selective than norbinaltorphimine (nor-BNI) for the κ opioid receptor in smooth muscle preparations.
Features and Benefits
This compound is featured on the Opioid Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Legal Information
Sold under US Patent No. 6,500,824.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
W C Stevens et al.
Journal of medicinal chemistry, 43(14), 2759-2769 (2000-07-14)
The indole moiety in the delta-opioid antagonist, naltrindole (2, NTI), was employed as a scaffold to hold an "address" for interaction with the kappa-opioid receptor. The attachment of the address to the 5'-position of the indole moiety was based on
Pathology and glia type specific changes of the DPP4 activity in the spinal cord contributes to the development and maintenance of hyperalgesia and shapes opioid signalling in chronic pain states
KiralyK, et al.
Scientific reports (2017)
R M Jones et al.
European journal of pharmacology, 396(1), 49-52 (2000-05-24)
5'-Guanidinonaltrindole (GNTI) possesses 5-fold greater opioid antagonist potency (K(e)=0.04 nM) and an order of magnitude greater selectivity (selectivity ratios >500) than the prototypical kappa-opioid receptor antagonist, norbinaltorphimine, in smooth muscle preparations. Binding and functional studies conducted on cloned human opioid
Pathology and glia type specific changes of the DPP4 activity in the spinal cord contributes to the development and maintenance of hyperalgesia and shapes opioid signalling in chronic pain states
KiralyK, et al.
Scientific Reports (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service