G2626
L-Glutamic Dehydrogenase from bovine liver
Type II, 50% glycerol solution, ≥35 units/mg protein
Synonym(s):
L-GLDH, L-Glutamate:NAD[P]+ Oxidoreductase (deaminating), Glutamate Dehydrogenase from bovine liver
About This Item
Recommended Products
biological source
bovine liver
type
Type II
form
glycerol solution (50%)
specific activity
≥35 units/mg protein
mol wt
310-350 kDa
UniProt accession no.
shipped in
wet ice
storage temp.
2-8°C
Gene Information
cow ... GLUD1(281785)
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
The bovine enzyme is characterized by three sets of properties:
- It has a reversible concentration-dependent association, producing higher molecular weight forms.
- Forms tight enzyme-reduced coenzyme-substrate ternary complexes whose rates of dissociation modulate the steady-state reaction rates.
- Exhibits a wide variety of effects from the binding of any of a number of nucleotide modifiers.
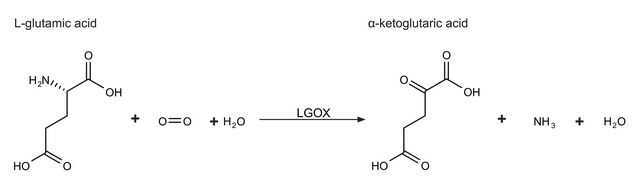
L-glutamic dehydrogenase catalyzes the conversion of glutamate to α-ketoglutarate.
Unit Definition
Physical form
Analysis Note
substrate
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
For use as a marker in SDS-PAGE; Albumin from chicken egg white, For use as a marker in SDS-PAGE; L-Lactic Dehydrogenase from rabbit muscle, Type XI, lyophilized powder, 600-1,200 units/mg protein
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service