C3515
Catalase from Aspergillus niger
ammonium sulfate suspension, ≥4,000 units/mg protein
Synonym(s):
H2O2:H2O2 oxidoreductase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
eCl@ss:
32160410
NACRES:
NA.54
Recommended Products
biological source
Aspergillus niger
Quality Level
form
ammonium sulfate suspension
specific activity
≥4,000 units/mg protein
mol wt
tetramer ~250 kDa
storage condition
(Tightly closed)
technique(s)
FISH: suitable
shipped in
wet ice
storage temp.
2-8°C
SMILES string
O(CC)C(=O)c1ccc(cc1)O
InChI
1S/C9H10O3/c1-2-12-9(11)7-3-5-8(10)6-4-7/h3-6,10H,2H2,1H3
InChI key
NUVBSKCKDOMJSU-UHFFFAOYSA-N
General description
Research area: Cell Signaling
Catalase is an active enzyme present in aerobic organisms. It is a ferric hemoprotein and a tetramer.
Catalase is an active enzyme present in aerobic organisms. It is a ferric hemoprotein and a tetramer.
Application
Catalase from Aspergillus niger has been used:
- as a positive control during the functional characterization of Clostridium difficile spore coat proteins.
- as a component of the catalase solution to prepare GLOX buffer with enzymes to maintain the embryos of Caenorhabditis elegans before single-molecule fluorescence in situ hybridization (smFISH) studies
- as a component of the imaging buffer for stochastic optical reconstruction microscopy (STORM) imaging of platelet-rich plasma
- as a supplement in Todd Hewitt media plus 0.5 % yeast extract (THY) media for the neutralization of pneumococcal H2O2
Biochem/physiol Actions
Catalase catalyzes the decomposition of hydrogen peroxide into water and oxygen. Each subunit of the tetramer contains iron bound to a protoheme IX group. The enzyme also strongly binds NADP, which is in close proximity to the heme group. Isoelectric point is found to be 6.5. Optimum pH for catalytic activity is 7.0. The enzyme activity is inhibited by 3-amino-1-H-1,2,4 triazole, cyanide, azide, hydroxylamine, cyanogen bromide, 2-mercaptoethanol, dithiothreitol, dianisidine, and nitrate. Incubation of catalase with ascorbate or ascorbate/Cu2+ results in degradation of the catalase molecule.
Catalase is involved in catalyzing the degradation of hydrogen peroxide (H2O2) to water and free oxygen. Catalase is used commercially for decomposing H2O2 as it is added as an antimicrobial agent in process streams. It also catalyzes the oxidation of electron donors like ethanol and phenols during lower concentrations of H2O2. Catalase shows antioxidant activity by protecting cells from higher levels of reactive oxygen species (ROS). Increased expression of catalase is observed in chronic lymphocytic leukemia, gastric cancer, glioma, and melanoma. Decreased levels of catalase are seen in acute myeloid leukemia, lung, pancreatic, prostate, and skin (non-melanoma) cancers. Hence catalase exhibits a dual role in cancer.
Caution
Freezing of solutions is not recommended.
Unit Definition
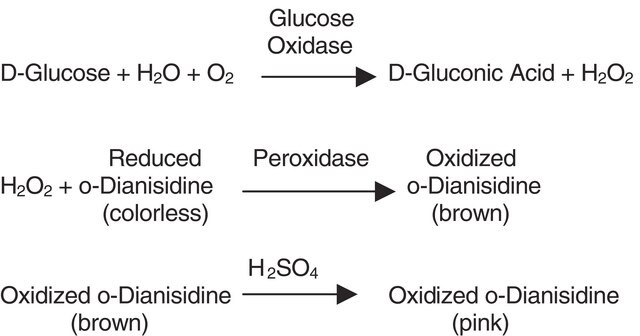
One unit will decompose 1.0 μmole of H2O2 per min at pH 7.0 at 25 °C, while the H2O2 concentration falls from 10.3 to 9.2 mM, measured by the rate of decrease of A240.
Physical form
Suspension in 3.2 M (NH4)2SO4 solution, pH 6.0
Analysis Note
Protein determined by biuret
antibody
Product No.
Description
Pricing
enzyme
Product No.
Description
Pricing
inhibitor
Product No.
Description
Pricing
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jacek Switala et al.
Archives of biochemistry and biophysics, 401(2), 145-154 (2002-06-11)
Catalases from 16 different organisms including representatives from all three phylogenetic clades were purified and characterized to provide a comparative picture of their respective properties. Collectively the enzymes presented a diverse range of activities and properties. Specific activities ranged from
Seleipiri Charles et al.
Analytical chemistry, 93(3), 1369-1376 (2020-12-24)
Recent development in fluorescence-based molecular tools has contributed significantly to developmental studies, including embryogenesis. Many of these tools rely on multiple steps of sample manipulation, so obtaining large sample sizes presents a major challenge as it can be labor-intensive and
3D structure modeling of catalase enzyme from Aspergillus Fumigatus
Dwivedi V D, et al.
Open journal of immunology, 1(1) (2016)
J Fiedurek et al.
Journal of applied microbiology, 89(1), 85-89 (2000-08-17)
A novel method for increasing dissolved oxygen concentration in culture media has been developed. It involves adding hydrogen peroxide (H2O2) to the medium which is then decomposed to oxygen and water by catalase. Some factors affecting oxygenation of culture were
L Brunelli et al.
Free radical biology & medicine, 30(7), 709-714 (2001-03-29)
Previously, we found that catalase enhanced the protection afforded by superoxide dismutase to Escherichia coli against the simultaneous generation of superoxide and nitric oxide (Brunelli et al., Arch. Biochem. Biophys. 316:327-334, 1995). Hydrogen peroxide itself was not toxic in this
Articles
Instructions for working with enzymes supplied as ammonium sulfate suspensions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service