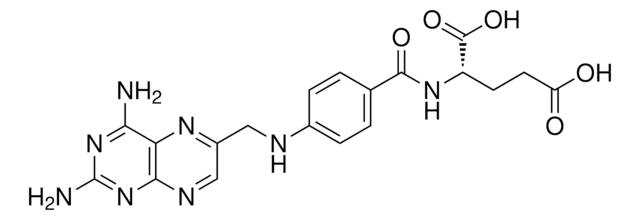
A1784
Aminopterin
powder
Synonym(s):
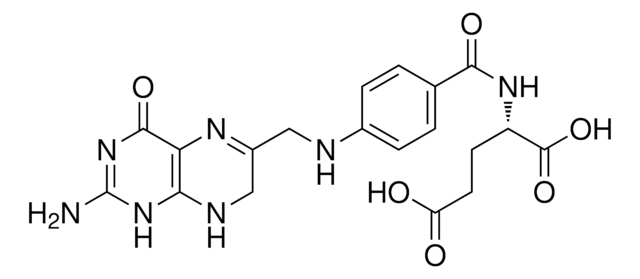
(S)-2-{4-[(2,4-Diaminopteridin-6-yl)methylamino]benzamido}pentanedioic acid, 4-Amino-PGA, 4-Aminofolic acid, 4-Aminopteroyl-L-glutamic acid
About This Item
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥97% (TLC)
form
powder
color
yellow
mp
225 °C
solubility
2 M NaOH: 50 mg/mL (Solutions may be stored for 1-2 days at 4°C.)
DMSO: soluble (Solutions may be stored for 1-2 days at 4°C.)
ε (extinction coefficient)
24,500 at 282 nm in 0.1 M NaOH at 1 M
25,700 at 261 nm in 0.1 M NaOH at 1 M
8,100 at 373 nm in 0.1 M NaOH at 1 M
storage temp.
−20°C
SMILES string
Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1
InChI
1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1
InChI key
TVZGACDUOSZQKY-LBPRGKRZSA-N
Gene Information
human ... FPGS(2356)
mouse ... Fpgs(14287)
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
Features and Benefits
Linkage
inhibitor
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Oral - Eye Irrit. 2 - Muta. 2 - Repr. 1B - Skin Irrit. 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
This issue of Biofiles reviews some of our newest and most innovative technologies and their specific applications toward cancer research. In preparing this issue of Biofiles, one is reminded how complex the disease of cancer is, and how difficult it is to identify one topic that is completely unrelated to any other.
Neoplastic cells are highly dependent on the de novo synthesis of nucleotides to maintain sufficient pools to support DNA replication and the production of RNA.
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service