45398
Chlorthiamid
PESTANAL®, analytical standard
Synonym(s):
2,6-Dichlorobenzenecarbothioamide, 2,6-Dichlorothiobenzamide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
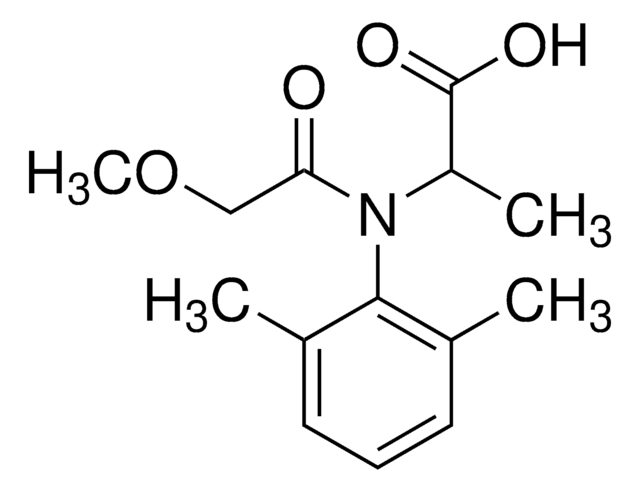
Empirical Formula (Hill Notation):
C7H5Cl2NS
CAS Number:
Molecular Weight:
206.09
Beilstein:
1910353
EC Number:
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
SMILES string
NC(=S)c1c(Cl)cccc1Cl
InChI
1S/C7H5Cl2NS/c8-4-2-1-3-5(9)6(4)7(10)11/h1-3H,(H2,10,11)
InChI key
KGKGSIUWJCAFPX-UHFFFAOYSA-N
Related Categories
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Legal Information
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
212.0 °F
Flash Point(C)
> 100 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J E Bakke et al.
Xenobiotica; the fate of foreign compounds in biological systems, 18(9), 1063-1075 (1988-09-01)
1. Twelve 14C-labelled metabolites were isolated from either urine or bile from either rats (11 metabolites) or goats (7 metabolites) given single oral doses of 2,6-dichlorobenzo[14C]nitrile (DCBN). Five of these metabolites were also excreted in urine from rats dosed orally
E B Brittebo et al.
Fundamental and applied toxicology : official journal of the Society of Toxicology, 17(1), 92-102 (1991-07-01)
The toxic effects of the herbicide chlorthiamid (2,6-dichlorothiobenzamide) and its major environmental metabolite 2,6-dichlorobenzamide (DCBA) were examined in the nasal passages of C57Bl mice following single ip injections. Chlorthiamid (12.25, and 50 mg/kg) induced an extensive destruction of the olfactory
L Bruun et al.
Journal of immunological methods, 240(1-2), 133-142 (2000-06-16)
2,6-Dichlorobenzamide (BAM) is the dominant degradation product in soil of the widely used herbicide dichlobenil. To detect BAM in water, a highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) was developed. As an alternative to conventional coating of ELISA plates
C Eriksson et al.
Toxicology letters, 76(3), 203-208 (1995-04-01)
Chlorthiamid (2,6-dichlorothiobenzamide) and its major metabolite 2,6-dichlorobenzonitrile are olfactory toxicants with a high in vivo covalent binding in the olfactory mucosa of mice. This study showed that the cytochrome P450 (P450) inhibitors, metyrapone and sodium-diethyldithiocarbamate, abolished the chlorthiamid-induced toxicity (12
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service