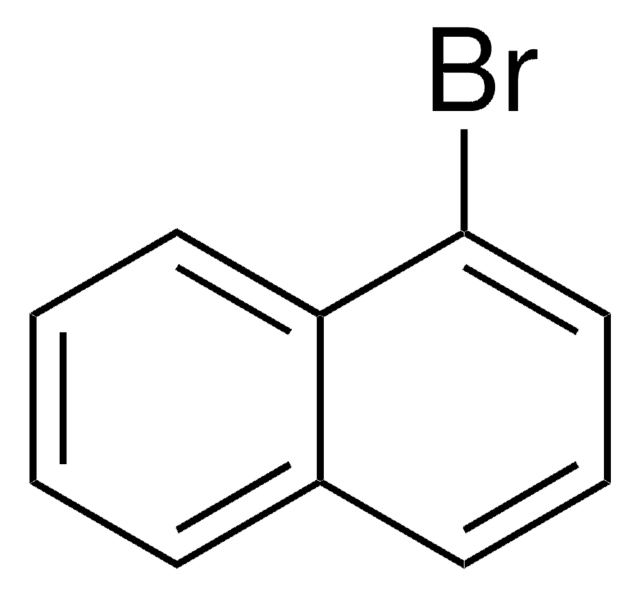
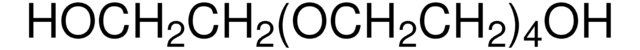158429
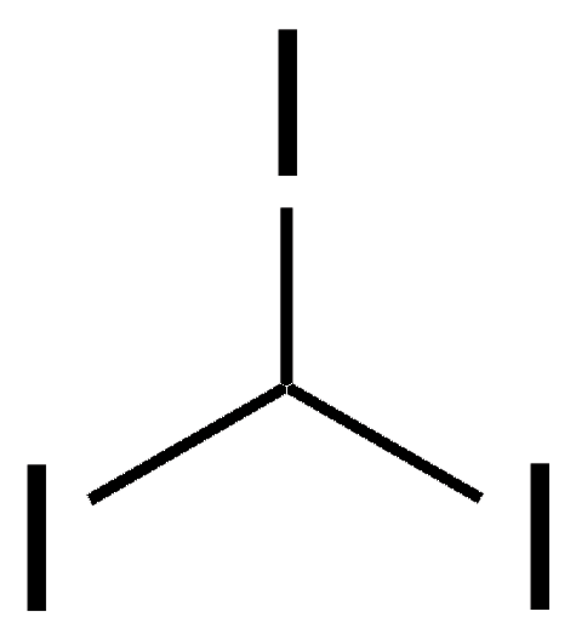
Diiodomethane
ReagentPlus®, 99%, contains copper as stabilizer
Synonym(s):
Methylene iodide
About This Item
Recommended Products
grade
reagent
Quality Level
vapor density
9.25 (vs air)
product line
ReagentPlus®
Assay
99%
form
liquid
contains
copper as stabilizer
bp
67-69 °C/11 mmHg (lit.)
mp
5-8 °C (lit.)
density
3.325 g/mL at 25 °C (lit.)
functional group
iodo
SMILES string
ICI
InChI
1S/CH2I2/c2-1-3/h1H2
InChI key
NZZFYRREKKOMAT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Diiodomethane (CH2I2) is an iodine containing organic compound. Its decomposition in acetonitrile initiated by 310nm light has been investigated. Femtosecond pump-probe spectroscopic and ab initio molecular dynamics simulations studies of the photodecomposition of CH2I2 suggest the formation of the isomer of diiodomethane (CH2I-I) as hot photoproduct. In the atmosphere, it undergoes photolysis in the presence of ozone to afford iodine oxide (IO) which results in the formation of aerosols. Its vacuum ultraviolet (VUV) photoabsorption spectrum has been reported.
Application
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service