E64-RO
Roche
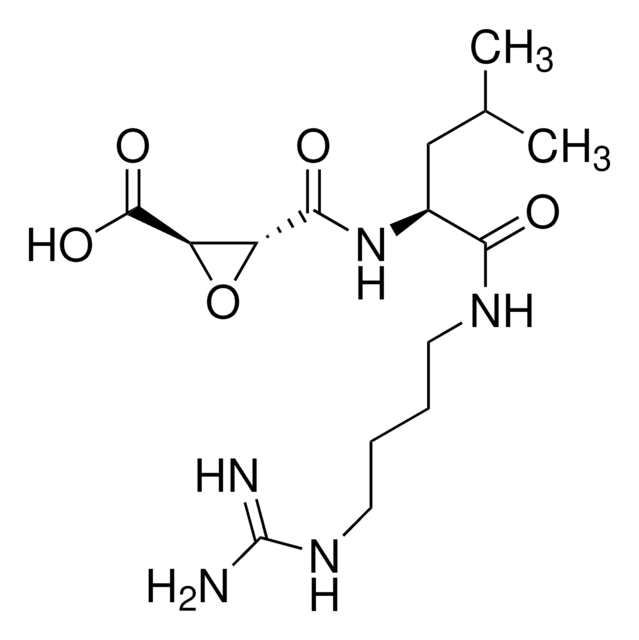
E-64
N-[N-(L-3-Trans-carboxirane-2-carbonyl)-L-leucyl]-agmatine
Synonym(s):
E-64, n-[n-( l-transepoxysuccinic acid)-l-leucyl]-agmatine, n-[n-(l-3-trans-carboxirane-2-carbonyl)-l-leucyl]-4-aminobutylguanidine, n-[n-(l-3-trans-carboxirane-2-carbonyl)-l-leucyl]-agmatine.
note: another name for l-3-trans-carboxyoxiran-2-carboxylic acid acid is l-transepoxysuccinic acid. agmatine is 4-aminobutylguanidine., trans-Epoxysuccinyl-L-leucylamido(4-guanidino)butane, L-trans-3-Carboxyoxiran-2-carbonyl-L-leucylagmatine, N-(trans-Epoxysuccinyl)-L-leucine 4-guanidinobutylamide
About This Item
Recommended Products
Quality Level
Assay
>95% (HPLC)
form
powder
mol wt
Mr 357.4
packaging
pkg of 10 mg (10874523001)
pkg of 25 mg (11585681001)
manufacturer/tradename
Roche
pH range
2-10
solubility
ethanol: water (1:1): soluble 20 mg/mL
storage temp.
2-8°C
SMILES string
CC(C)C[C@H](NC(=O)[C@@H]1O[C@H]1C(O)=O)C(=O)NCCCCNC(N)=N
InChI
1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10+,11+/m0/s1
InChI key
LTLYEAJONXGNFG-HBNTYKKESA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Specificity
Application
It has been used in the affinity chromatography for the purification of hemoglobin receptors.
The inhibition of thiol proteases by E-64 appears to be of a non-competitive nature between the SH components. The inhibition is also irreversible, and is altered after gel filtration (Sephadex column) or dialysis after incubation of papain with E-64. The enzyme and inhibitor combine in an equimolar ratio.
Biochem/physiol Actions
Preparation Note
Working solution: Soluble to 20 mg/ml (stock solution) in a 1:1 (v/v) mixture of ethanol and water (vortexing or slight warming in water bath (40 °C) may facilitate dissolution). Also soluble in a neutral water/methanol solution, in water, methanol, acetic acid, pyridine and DMSO. Sparingly soluble in ethanol and propanol. Insoluble in acetone, chloroform, ethyl ether and benzene.
Storage conditions (working solution): -15 to -25 °C
Solutions are stable for one month when stored in aliquots at -15 to -25 °C.
Reconstitution
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
STOT SE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
does not flash
Flash Point(C)
does not flash
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service