700052P
Avanti
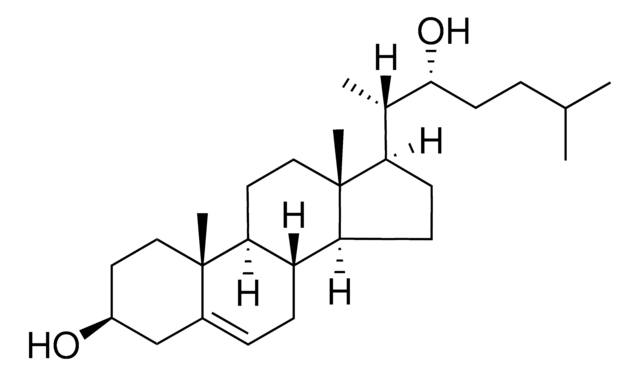
22(R)-hydroxycholesterol-d7
Avanti Polar Lipids
Synonym(s):
25,26,26,26,27,27,27-heptadeuterocholest-5-ene-3β,22R-diol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C27H39O2D7
CAS Number:
Molecular Weight:
409.70
UNSPSC Code:
41141804
NACRES:
NA.25
Recommended Products
description
cholest-5-ene-3β,22(R)-diol-d7
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 1 mg (700052P-1mg)
manufacturer/tradename
Avanti Polar Lipids
application(s)
lipidomics
metabolomics
shipped in
dry ice
storage temp.
−20°C
General description
22(R)-hydroxycholesterol is a diastereomer of 22(S)-hydroxycholesterol. It is present in the neonate brain. 22(R)-hydroxycholesterol-d7 is a deuterated form of 22(R)-hydroxycholesterol.
Biochem/physiol Actions
22(R)-hydroxycholesterol (22(R)-HC) is a liver X receptor (LXR) ligand and is a key for the receptor activation.. 22(R)-HC promotes mesenchymal stem cell osteogenesis along with other oxysterols. It also regulates cholesterol homeostasis and suppresses prostate tumor progression. Low levels of 22(R)-HC is observed in Alzheimer′s disease and may be implicated in neuroinflammation and neurodegenerative diseases.
Packaging
5 mL Amber Glass Screw Cap Vial (700052P-1mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hyunmi Kim et al.
Biochimica et biophysica acta, 1859(8), 1056-1070 (2016-05-22)
MAP kinase phosphatase (MKP)-1 plays a pivotal role in controlling MAP kinase (MAPK)-dependent (patho) physiological processes. Although MKP-1 gene expression is tightly regulated at multiple levels, the underlying mechanistic details remain largely unknown. In this study, we demonstrate that MKP-1
Megan M Augustin et al.
The Plant journal : for cell and molecular biology, 82(6), 991-1003 (2015-05-06)
Steroid alkaloids have been shown to elicit a wide range of pharmacological effects that include anticancer and antifungal activities. Understanding the biosynthesis of these molecules is essential to bioengineering for sustainable production. Herein, we investigate the biosynthetic pathway to cyclopamine
Chih-Pin Chuu et al.
Anticancer research, 30(9), 3643-3648 (2010-10-15)
Previously, we and other groups reported that liver X receptor (LXR) agonists T0901317, 22(R)-hydroxycholesterol, and 24(S)-hydroxycholesterol suppressed the proliferation of prostate and breast cancer cells. In this study, we report that T0901317 and 22(R)-hydroxycholesterol treatment inhibited the proliferation of different
ApoA-I or ABCA1 expression suppresses fatty acid synthesis by reducing 27-hydroxycholesterol levels.
Donglin Ma et al.
Biochimie, 103, 101-108 (2014-05-06)
Abnormal lipid metabolism may contribute to the pathogenesis of non-alcoholic steatohepatitis (NASH). The ATP-binding cassette transporter A1 (ABCA1) protein mediates the transport of cholesterol and phospholipids from cells to apolipoprotein A-I (apoA-I) to generate nascent HDL particles. Previous studies revealed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service