T67806
Triisopropyl phosphite
95%
Synonym(s):
Isopropyl phosphite, Tri-2-propyl phosphite, Triisopropoxyphosphine, Tris(isopropyl) phosphite
About This Item
Recommended Products
vapor pressure
<2 mmHg ( 20 °C)
Quality Level
Assay
95%
refractive index
n20/D 1.411 (lit.)
bp
63-64 °C/11 mmHg (lit.)
density
0.844 g/mL at 25 °C (lit.)
SMILES string
CC(C)OP(OC(C)C)OC(C)C
InChI
1S/C9H21O3P/c1-7(2)10-13(11-8(3)4)12-9(5)6/h7-9H,1-6H3
InChI key
SJHCUXCOGGKFAI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- With Ru-based indenylidene complexes to form 1st generation complexes for metathesis reactions.
- In Perkow-type reaction to synthesize compounds containing polarized carbon-carbon double bonds.
- For the synthesis of phosphonohydrazines by reacting with arylamines and isoamyl nitrite.
It can also be used as an alternative to triphenylphosphine in Mitsunobu reaction to facilitate the isolation of products.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
154.4 °F - closed cup
Flash Point(C)
68 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service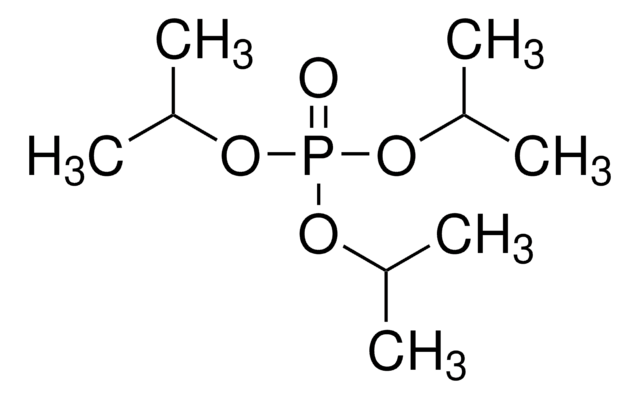



![2-[(Acetoxymethyl)allyl]trimethylsilane 99%](/deepweb/assets/sigmaaldrich/product/structures/162/702/2145c2ca-c80b-47c6-abf6-d030858f0cc2/640/2145c2ca-c80b-47c6-abf6-d030858f0cc2.png)