About This Item
Recommended Products
vapor density
3.4 (vs air)
vapor pressure
0.16 mmHg ( 20 °C)
Assay
99%
autoignition temp.
870 °F
expl. lim.
7.1 %
bp
200 °C (lit.)
mp
51-56 °C (lit.)
SMILES string
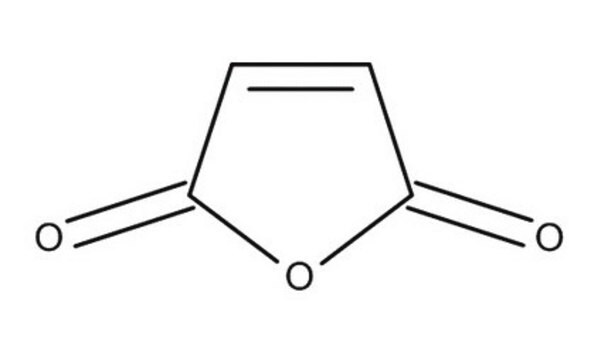
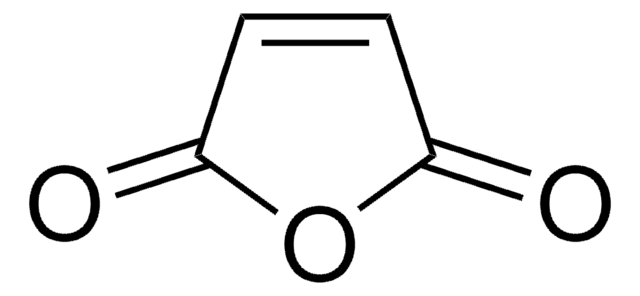
O=C1OC(=O)C=C1
InChI
1S/C4H2O3/c5-3-1-2-4(6)7-3/h1-2H
InChI key
FPYJFEHAWHCUMM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The structure of maleic acid consists of four carbon molecules along with carboxylate groups on either ends, with a double bond between the central carbon atoms. The anhydride of maleic acid has five atoms in its cyclic molecule, the unsaturated bond undergoes free radical polymerization in the presence of an initiator.
Application
- In the grafting process of high-density polyethylene (HDPE) by the monomer microencapsulation technique, which enables the introduction of enhanced functionality and improved properties to the polymer for functional coatings, membranes, or surface treatments, adhesives, and coatings.
- In the functionalization of polylactic acid (PLA) and acts as a coupling agent in natural fiber biocomposites. The functionalization improves the compatibility and interfacial adhesion between PLA and natural fibers, leading to enhanced mechanical properties and expanded applications of the resulting biocomposite materials.
- Maleic anhydride may be used in the synthesis of unsaturated polyester resins and as a reactant in synthesizing important products such as agricultural chemicals, lubricant additives, and food acidulatents.
Physical form
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1A - STOT RE 1 Inhalation
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
217.4 °F - closed cup
Flash Point(C)
103 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Molecular Layer Deposition of Organic and Hybrid Organic-Inorganic Polymers
By altering the physicochemical properties, smart or intelligent drug delivery systems can be designed to deliver therapeutic molecules on-demand. Learn more about the application of stimuli-responsive materials in drug delivery.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service