All Photos(1)
About This Item
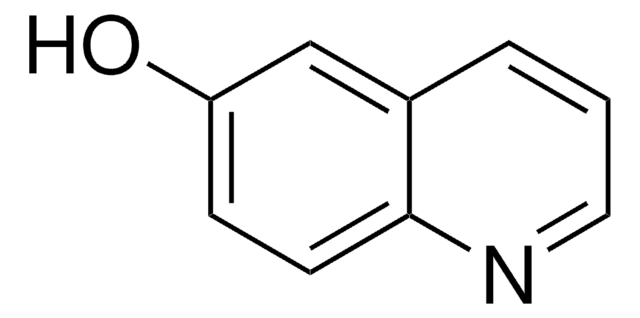
Empirical Formula (Hill Notation):
C9H7NO
CAS Number:
Molecular Weight:
145.16
Beilstein:
2900
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
200-202 °C (lit.)
SMILES string
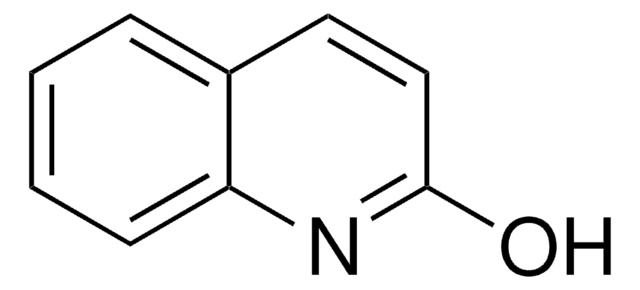
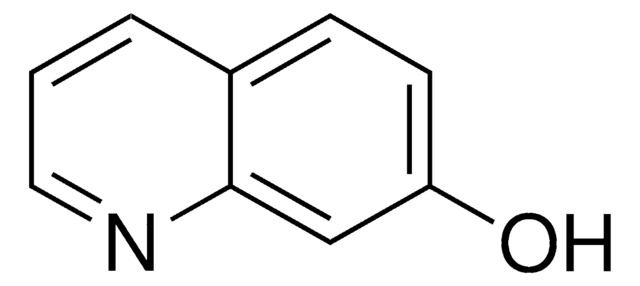
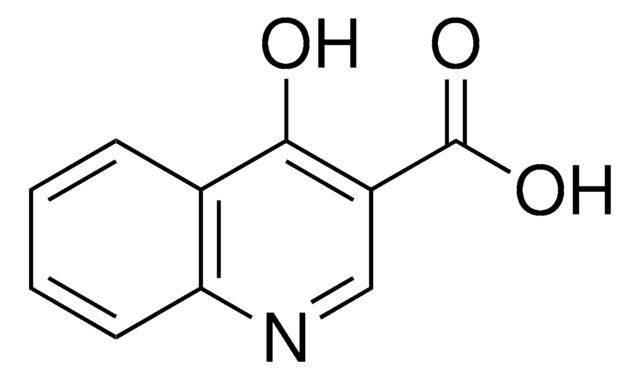
Oc1ccnc2ccccc12
InChI
1S/C9H7NO/c11-9-5-6-10-8-4-2-1-3-7(8)9/h1-6H,(H,10,11)
InChI key
PMZDQRJGMBOQBF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Quinolinol (4-quinolone) is a quinolone compound which forms the core moiety of antibacterials such as norfloxacin, nalidixic acid, ciprofloxacin and cinoxacin.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Charinya Khamphukdee et al.
Molecules (Basel, Switzerland), 23(9) (2018-09-12)
The previously unreported flavone glycoside, demethyltorosaflavone B (2) and the E-propenoic acid substituted flavone, torosaflavone E (3a), were isolated together with nine previously reported metabolites, including indole-3-carbaldehyde, oleanonic acid, vanillic acid, p-hydroxybenzoic acid, altheranthin (1a), alternanthin B (1b), demethyltorosaflavone D
Josip Podobnik et al.
Biomolecules, 10(11) (2020-11-19)
Juvenile delinquency is related to several biological factors, yet very few vulnerability biomarkers have been identified. Previous data suggest that the enzyme monoamine oxidase B (MAO-B) influences several personality traits linked to the propensity to engage in delinquent behavior. Building
T R Hall et al.
Comparative biochemistry and physiology. C: Comparative pharmacology, 71(2), 141-144 (1982-01-01)
1. Perch brain homogenates were incubated in vitro and monoamine oxidase (MAO) activity was determined fluorometrically, using a kynuramine substrate. 2. Clorgyline, harmaline and deprenyl inhibited MAO activity in a concentration-related manner, with single sigmoid inhibition curves, and the type
Yan-Li Gai et al.
Inorganic chemistry, 51(24), 13128-13137 (2012-12-05)
A series of novel two-dimensional (2D) lanthanide coordination polymers with 4-hydroxyquinoline-2-carboxylate (H(2)hqc) ligands, [Ln(Hhqc)(3)(H(2)O)](n)·3nH(2)O (Ln = Eu (1), Tb (2), Sm (3), Nd (4), and Gd (5)) and [Ln(Hhqc)(ox)(H(2)O)(2)](n) (Ln = Eu (6), Tb (7), Sm (8), Tm (9), Dy
Thomas Rahn et al.
Organic & biomolecular chemistry, 7(9), 1931-1938 (2009-07-11)
The reaction of 1-methoxy-1,3-bis(trimethylsilyloxy)-1,3-butadiene with 2-methoxybenzoyl chlorides afforded 3,5-diketoesters which were transformed, by treatment with boron tribromide, into functionalized 2-hydroxychroman-4-ones or chromones. The reaction of 1-methoxy-1,3-bis(trimethylsilyloxy)-1,3-butadiene with 2-nitrobenzoyl chlorides and subsequent reduction of the nitro group afforded functionalized 4-hydroxyquinolines. Their
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service