C104507
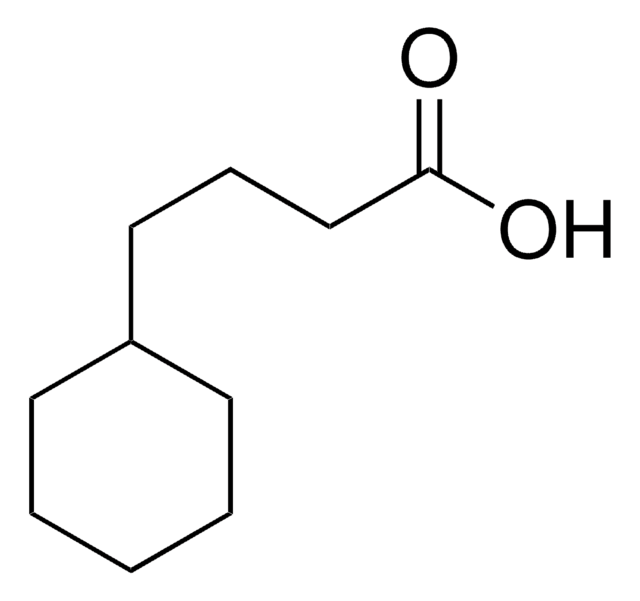
Cyclohexaneacetic acid
≥99%
Synonym(s):
Cyclohexylacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H11CH2CO2H
CAS Number:
Molecular Weight:
142.20
Beilstein:
2041326
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
form
solid
refractive index
n20/D 1.463 (lit.)
bp
242-244 °C (lit.)
mp
28-32 °C (lit.)
density
1.007 g/mL at 25 °C (lit.)
SMILES string
OC(=O)CC1CCCCC1
InChI
1S/C8H14O2/c9-8(10)6-7-4-2-1-3-5-7/h7H,1-6H2,(H,9,10)
InChI key
LJOODBDWMQKMFB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J E Tulliez et al.
Lipids, 16(12), 888-892 (1981-12-01)
Among the urinary metabolites of dodecylcyclohexane or cyclohexylacetic acid, the glycine conjugate of 1-hydroxy-cyclohexylacetic acid was identified and its origin studied, using cyclohexylacetic acid as the starting molecule, as it results from beta-oxidation of cyclohexyldodecanoic acid produced by terminal oxidation
Hiroaki Iwaki et al.
Current microbiology, 57(2), 107-110 (2008-04-09)
Six cyclohexylacetic acid-degrading strains were isolated from soil samples in Japan and identified as members of the genera Cupriavidus (strain KUA-1), Rhodococcus, and Dietzia by 16S rRNA gene sequence analysis. For the first time members of these genera were shown
Rabea Schlüter et al.
Applied microbiology and biotechnology, 103(10), 4137-4151 (2019-04-04)
The cycloalkanes, comprising up to 45% of the hydrocarbon fraction, occur in crude oil or refined oil products (e.g., gasoline) mainly as alkylated cyclohexane derivatives and have been increasingly found in environmental samples of soil and water. Furthermore, short-chain alkylated
H J Ougham et al.
Journal of bacteriology, 150(3), 1172-1182 (1982-06-01)
A strain of Arthrobacter was isolated by enrichment culture with cyclohexaneacetate as the sole source of carbon and grew with a doubling time of 4.2 h. In addition to growing with cyclohexaneacetate, the organism also grew with cyclohexanebutyrate at concentrations
Dean M Quesnel et al.
Chemosphere, 84(4), 504-511 (2011-04-05)
Naphthenic acids (NAs) are a major contributor to toxicity in tailings waste generated from bitumen production in the Athabasca Oil Sands region. While investigations have shown that bacteria can biodegrade NAs and reduce tailings toxicity, the potential of algae to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service