B75956
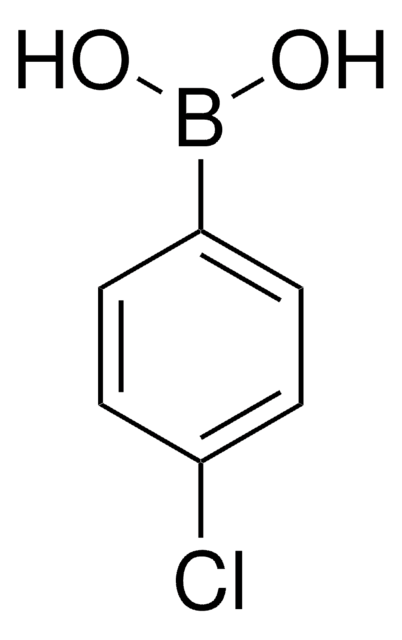
4-Bromophenylboronic acid
≥95.0%
Synonym(s):
(p-Bromophenyl)boronic acid, 4-Bromobenzeneboronic acid, 4-Bromophenylboric acid, p-Bromobenzeneboronic acid, p-Bromophenylboric acid, NSC 25407
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4B(OH)2
CAS Number:
Molecular Weight:
200.83
Beilstein:
2936347
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
95%
form
crystals
mp
284-288 °C (lit.)
SMILES string
OB(O)c1ccc(Br)cc1
InChI
1S/C6H6BBrO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
InChI key
QBLFZIBJXUQVRF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Reagent used for
Reagent used in Preparation of
- Palladium catalyzed Suzuki-Miyaura cross-couplings
- Pd(II)-catalyzed diastereoselective conjugate additions
- Palladium-catalyzed stereoselective Heck-type reaction of allylic esters with arylboronic acids
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides
- Pd-catalyzed arylative cyclization of alkyne-tethered enals or enones via carbopalladation of alkynes
- Copper-catalyzed cross-couplings
Reagent used in Preparation of
- Gallate-based obovatol analogs with potential anti-tumor activity
- Protein modulators and enzymatic and kinase inhibitors
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
James A Jordan-Hore et al.
Organic letters, 14(10), 2508-2511 (2012-05-02)
Ligand-free cationic Pd(II) catalyst with NaNO3 as an additive is a highly active catalytic system for conjugate additions to sterically hindered γ-substituted cyclohexenones. More challenging γγ- and βγ-substrates also react well to produce products with quaternary centers in good dr.
Synthesis of obovatol derivatives and their preliminary evaluation as antitumor agents
Lee, M.-S.; et al.
Bull. Korean Chem. Soc., 28, 1601-1604 (2007)
Tahlia R Meola et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 129, 145-153 (2018-06-02)
The synergistic effect of nanosizing and lipid-based drug delivery systems (LBDDS) was explored to enhance formulation drug loading levels and improve drug solubilisation in the gastrointestinal environment. A novel formulation combining drug nanocrystals and silica-lipid hybrid (SLH) microparticles as a
Chuan Xiao et al.
Bioorganic & medicinal chemistry, 19(23), 7100-7110 (2011-11-01)
A series of purine nucleoside analogues bearing an aryl and hetaryl group in position 6 were prepared and their biological activities were assessed by in vitro CDK1/Cyclin B1 and CDK2/Cyclin A2 kinase assay. From the synthesized chemicals, three Xylocydine derivatives
Erin Tay et al.
Pharmaceutics, 12(1) (2019-12-28)
Lipid based formulations (LBFs) are commonly employed to enhance the absorption of highly lipophilic, poorly water-soluble drugs. However, the utility of LBFs can be limited by low drug solubility in the formulation. Isolation of ionizable drugs as low melting, lipophilic
Global Trade Item Number
| SKU | GTIN |
|---|---|
| B75956-1G | |
| B75956-5G | 4061833441992 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service