B4007
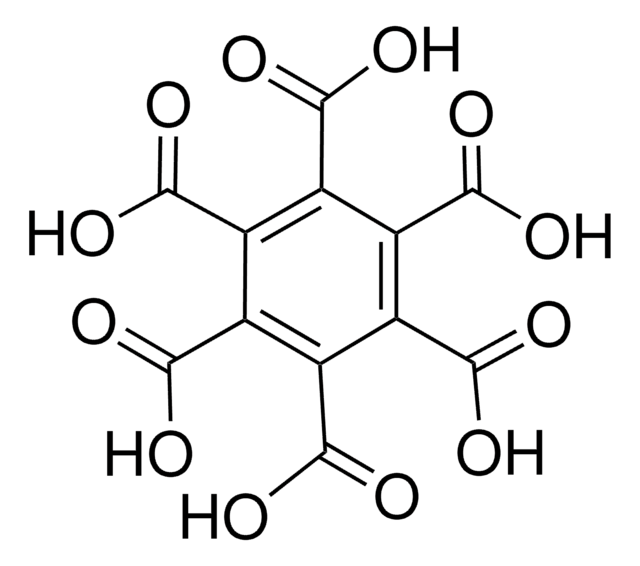
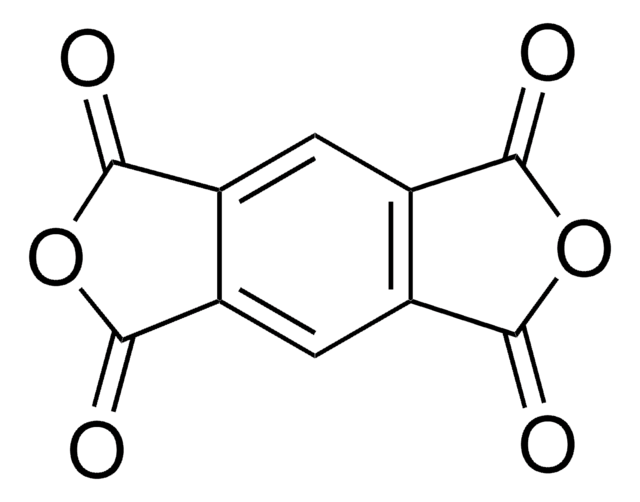
1,2,4,5-Benzenetetracarboxylic acid
96%
Synonym(s):
Pyromellitic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H2(CO2H)4
CAS Number:
Molecular Weight:
254.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
powder
mp
281-284.5 °C (lit.)
SMILES string
OC(=O)c1cc(C(O)=O)c(cc1C(O)=O)C(O)=O
InChI
1S/C10H6O8/c11-7(12)3-1-4(8(13)14)6(10(17)18)2-5(3)9(15)16/h1-2H,(H,11,12)(H,13,14)(H,15,16)(H,17,18)
InChI key
CYIDZMCFTVVTJO-UHFFFAOYSA-N
Related Categories
Application
1,2,4,5-Benzenetetracarboxylic acid is extensively used as a ligand in the synthesis of a wide range of metal-organic frameworks (MOFs) and coordination polymers. It can also be used to prepare supramolecular hydrogels by synthesizing gelators reacting 1,2,4,5-benzenetetracarboxylic acid with 4-hydroxy pyridine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
617.0 °F
Flash Point(C)
325 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Chattopadhyay et al.
Journal of chromatography. A, 758(2), 255-261 (1997-01-17)
A capillary electrophoresis (CE) method was developed for determining the diol content of supports used in high-performance affinity or size exclusion chromatography. This method involved oxidizing the diol-bonded support with periodate, followed by the use of CE to separate and
Yauhen Y Karabach et al.
Journal of inorganic biochemistry, 102(5-6), 1190-1194 (2008-01-08)
The new inorganic 1D coordination polymer [Cu2(H3tea)2(mu4-pma)]n has been prepared, via self-assembly in aqueous medium, from copper(II) nitrate, triethanolamine (H3tea), pyromellitic acid (H4pma) and lithium hydroxide, and characterized by IR spectroscopy, elemental and single-crystal X-ray diffraction analyses. This compound and
Gustavo A Blanco-Heras et al.
Journal of chromatography. A, 1144(2), 275-278 (2007-02-13)
Selectivity and robustness of the pyromellitic acid (PMA) based background electrolyte was improved in order to increase its applicability for routine analysis of inorganic and organic anions in real samples. An electrolyte composed of 6.75 mM PMA, 0.5 mM hexamethonium
James E A Webb et al.
Journal of the American Chemical Society, 129(22), 7155-7162 (2007-05-15)
The N,N',N'',N'''-1,2,4,5-tetra(ethylhexanoate) pyromellitamide is found to be capable of both intermolecular aggregation and binding to small anions. It is synthesized by aminolysis of pyromellitic anhydride with ethanolamine, followed by a reaction with hexanoyl chloride. The single-crystal X-ray structure of the
K-J Westin et al.
Journal of colloid and interface science, 282(2), 359-369 (2004-12-14)
The influence of four calcium complexing substances, i.e., citric acid (CIT), diethylenetriaminepentaacetic acid (DTPA), ethylenediaminetetraacetic acid (EDTA) and pyromellitic acid (PMA), on the crystal growth rate of the calcium carbonate polymorphs aragonite and calcite has been studied. Using a seeded
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service