911852
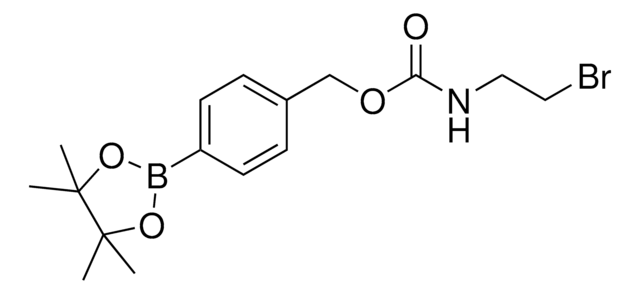
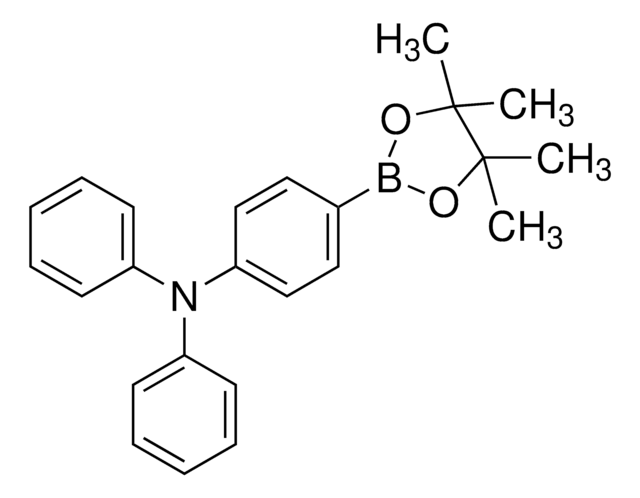
4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl (2-aminoethyl)carbamate
≥95%
Synonym(s):
Aminoethyl boronic acid pinacol ester nuclear tag, Benzyl boronate tag, Nucleus-targeting probe building block
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C16H25BN2O4
Molecular Weight:
320.19
UNSPSC Code:
12352101
Recommended Products
Assay
≥95%
form
powder or solid
storage temp.
2-8°C
Application
This benzyl boronate tag is a synthetic means for the subcellular targeting of cargo to the nucleus. Intracellular targeting can be important for understanding the localization of metabolites, proteins, or chemical probes or to increase therapeutic efficacy by concentrating a drug at its site of action and reducing off-target effects. Localization specifically to the nucleus is typically achieved using peptide localization signals and/or relies on passive diffusion. It was recently demonstrated, however, that nuclear targeting could be accomplished instead with a small-molecule motif benzyl boronic acid via synergistic active and passive transport processes. Tang, et al, presented examples using this tag to deliver proteins to the nucleus by the importin α/β pathway, including fluorescent proteins, ribonuclease A (RNase A), and chymotrypsin. The conjugation of this nucleus-targeting building block to other proteins or small molecules will facilitate nuclear localization in varied chemical biology experiments.
related product
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Rui Tang et al.
Journal of the American Chemical Society, 139(25), 8547-8551 (2017-06-10)
Active intracellular transport is a central mechanism in cell biology, directed by a limited set of naturally occurring signaling peptides. Here, we report the first nonpeptide moiety that recruits intracellular transport machinery for nuclear targeting. Proteins synthetically modified with a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service