745537
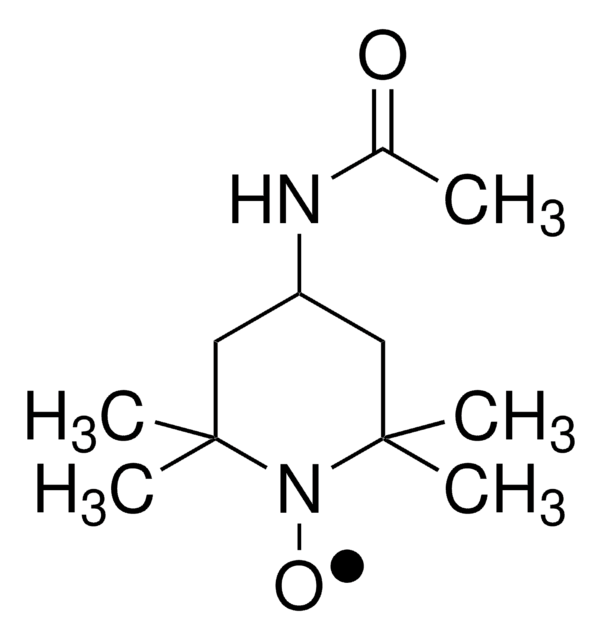
4-(Acetylamino)-2,2,6,6-tetramethyl-1-oxo-piperidinium tetrafluoroborate
97% (HPLC)
Synonym(s):
4-Acetamido-2,2,6,6-tetramethyl-1-oxopiperidinium tetrafluoroborate, Bobbitt′s Salt
About This Item
Recommended Products
Quality Level
Assay
97% (HPLC)
form
solid
reaction suitability
reagent type: oxidant
mp
191-197 °C (decomposition)
functional group
amide
storage temp.
2-8°C
SMILES string
F[B-](F)(F)F.CC(=O)NC1CC(C)(C)[N+](=O)C(C)(C)C1
InChI
1S/C11H20N2O2.BF4/c1-8(14)12-9-6-10(2,3)13(15)11(4,5)7-9;2-1(3,4)5/h9H,6-7H2,1-5H3;/q;-1/p+1
InChI key
HTMHEICBCHCWAU-UHFFFAOYSA-O
Related Categories
General description
Application
- Oxidation of alcohols to their concomitant aldehyde, ketone or carboxylic acid.
- Conversion of aldehydes to hexafluoroisopropyl (HFIP) esters via oxidative esterification.,·
- Deprotection of allyl ethers to corresponding aldehydes.
- Preparation of α,β-unsaturated ketones by dehydrogenation of perfluoroalkyl ketones.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 745537-25G | 4061833399859 |
| 745537-5G | 4061833339480 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)