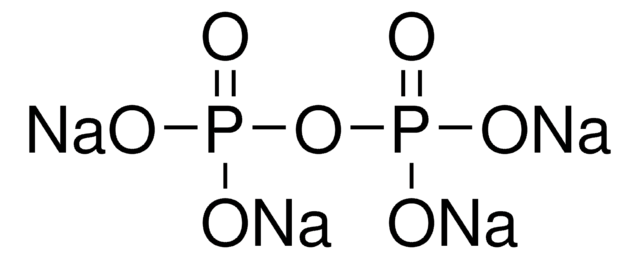71600
Sodium hexametaphosphate
65-70% P2O5 basis
Synonym(s):
SHMP, Calgon, Phosphate glass, water soluble, Polyphosphate sodium salt, Sodium polyphosphate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
form
solid
concentration
65-70% P2O5
InChI
1S/2Na.H4O7P2/c;;1-8(2,3)7-9(4,5)6/h;;(H2,1,2,3)(H2,4,5,6)/q2*+1;/p-2
InChI key
GYQBBRRVRKFJRG-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Sodium hexametaphosphate is an inorganic polyphosphate salt commonly used as a corrosion inhibitor, emulsifying agent and as a tooth whitening agent in dentifrice formulations.
Application
Sodium hexametaphosphate has been used as a deflocculant to prepare clay suspensions.
Biochem/physiol Actions
Intended for the cytologic demonstration of Naphthol AS-D Chloroacetate Esterase in blood, bone marrow films or tissue touch preparations.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Structure and properties of binder gels formed in the system Mg (OH) 2?SiO 2?H 2 O for immobilisation of Magnox sludge.
Walling S A, et al.
Dalton Transactions, 44(17), 8126-8137 (2015)
The role of sodium hexametaphosphate in the dissolution process of kaolinite and kaolin.
Andreola F, et al.
J. Eur. Ceram. Soc., 24(7), 2113-2124 (2004)
Extrinsic stain removal with a sodium hexametaphosphate-containing dentifrice: comparisons to marketed controls.
Gerlach R, et al.
The Journal of Clinical Dentistry, 13(1), 10-14 (2002)
Akiko Tanaka et al.
Biological & pharmaceutical bulletin, 40(2), 212-219 (2017-02-06)
The effect of changes in the mucosal fluid volume on the nasal drug absorption of powder formulations was evaluated using warfarin (WF), piroxicam (PXC), and norfloxacin (NFX) as model drugs. Lactose and sodium chloride (NaCl), which are water soluble and
Final report on the safety assessment of Sodium Metaphosphate, Sodium Trimetaphosphate, and Sodium Hexametaphosphate.
Lanigan R S.
International Journal of Toxicology, 20, 75-89 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service