661376
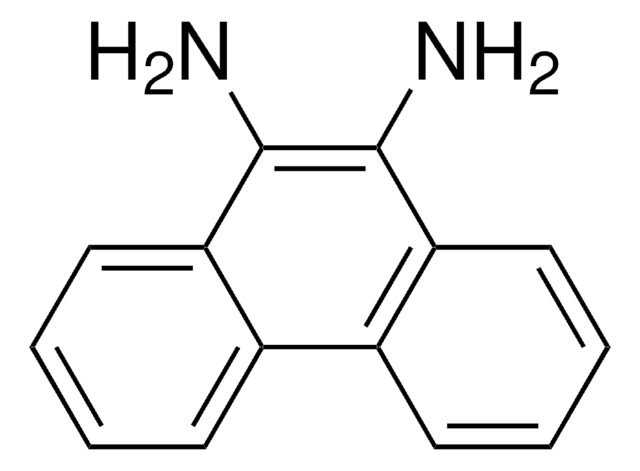
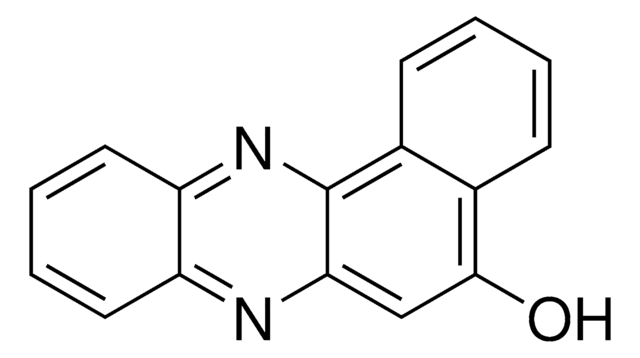
2,3-Diaminophenazine
90%
Synonym(s):
2,3-Phenazinediamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H10N4
CAS Number:
Molecular Weight:
210.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
90%
form
solid
mp
>300 °C
SMILES string
Nc1cc2nc3ccccc3nc2cc1N
InChI
1S/C12H10N4/c13-7-5-11-12(6-8(7)14)16-10-4-2-1-3-9(10)15-11/h1-6H,13-14H2
InChI key
VZPGINJWPPHRLS-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Sylvestre et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 96, 401-412 (2012-06-23)
Vibrational analysis of the planar electron-rich heterocyclic 2,3-diaminophenazine (DAP) molecule was carried out using FT-IR and FT-Raman spectroscopic techniques. The equilibrium geometry, harmonic vibrational wavenumbers, various bonding features have been computed using density functional method. The calculated molecular geometry parameters
Yuan-liang Jiang et al.
Guang pu xue yu guang pu fen xi = Guang pu, 22(3), 436-440 (2003-08-27)
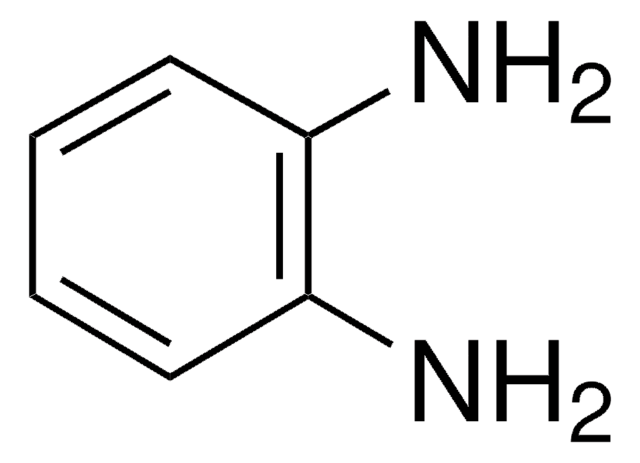
In this paper, we study the enzyme catalysis reaction kinetics that 2,3-diaminophenazine compound was synthesized with the horseradish peroxidase catalyzing reaction of H2O2 oxidizing o-phenylemediamine. First, the product of oxidation of o-phenylenediamine (OPD) by H2O2 catalyzed and by horseradish peroxidase
A Cebulska-Wasilewska et al.
Mutation research, 446(1), 57-65 (1999-12-29)
This paper presents studies on the genotoxicity of two aminophenazines: 2,3-diaminophenazine (DAP) and 2-amino-3-hydroxyphenazine (AHP). The genotoxic activities of these compounds were evaluated with human lymphocytes using the alkaline single cell gel electrophoresis (SCGE) assay and two cytogenetic assays (chromosome
Aihui Liang et al.
Journal of fluorescence, 18(6), 1035-1041 (2008-02-09)
Gold nanoparticles in size of 15 nm exhibit a resonance scattering (RS) peak at 580 nm, in pH 6.4 citrate buffer solutions. Horseradish peroxidase (HRP) strongly catalyzed the H2O2 oxidation of o-phenylenediamine to form an intergradation of cyclohexa-3, 5-diene-1, 2-diylidenediamine
Qi Zheng et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 61(6), 1035-1038 (2005-03-03)
A new method has been developed for the determination of myoglobin (Mb) based on its enzymatic activity for the oxidation of o-phenylenediamine (OPDA) with hydrogen peroxide. Stopped-flow spectrophotometry was used to study the kinetic behavior of the oxidation reaction. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service