All Photos(3)
About This Item
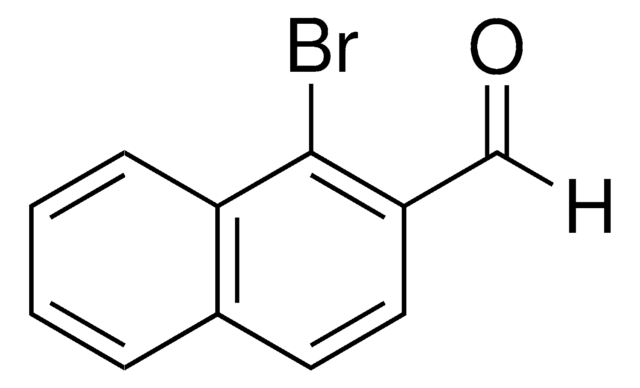
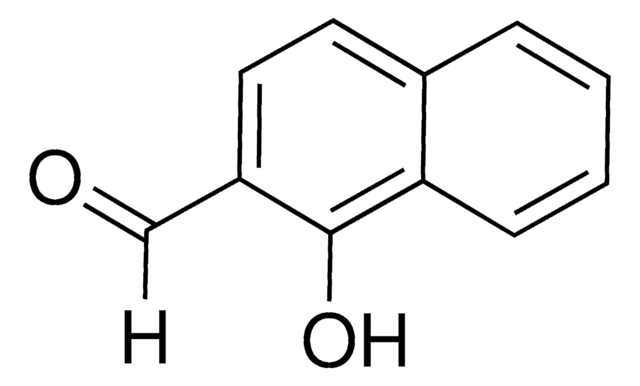
Linear Formula:
O2NC10H6CHO
CAS Number:
Molecular Weight:
201.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
109-110 °C (lit.)
functional group
aldehyde
nitro
SMILES string
[O-][N+](=O)c1c(C=O)ccc2ccccc12
InChI
1S/C11H7NO3/c13-7-9-6-5-8-3-1-2-4-10(8)11(9)12(14)15/h1-7H
InChI key
XQIMHJNMEFIADP-UHFFFAOYSA-N
General description
On irradiation with UV light, 1-nitro-2-naphthaldehyde gets transformed into the corresponding nitroso acid.
Application
1-Nitro-2-naphthaldehyde (NAA) may be used to prepare the precursors required for the preparation of 3-acetoxy-2-methylene-3-(1-nitronaphth-2-yl)propanoate and ethyl 3-acetoxy-2-methylene-3-(1-nitronaphth-2-yl)propanoate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Materials and systems for two photon 3-D ROM devices.
Dvornikov AS, et al.
IEEE Transactions on Components and Packaging Technologies, 20(2), 203-212 (1997)
Deevi Basavaiah et al.
Organic letters, 9(13), 2453-2456 (2007-06-01)
The Baylis-Hillman acetates have been conveniently transformed into tri-/tetracyclic heterocyclic frameworks containing an important azocine moiety via one-pot multistep protocol involving alkylation, reduction, and cyclization sequence.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service