All Photos(1)
About This Item
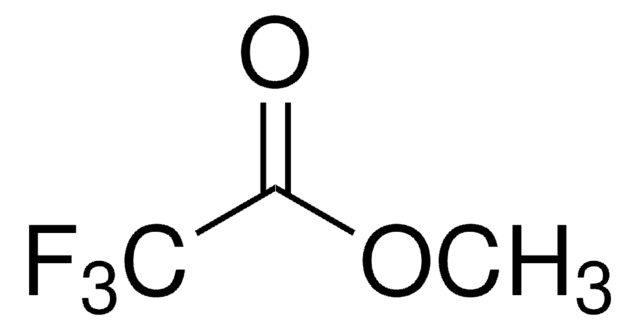
Linear Formula:
CF3COCO2C2H5
CAS Number:
Molecular Weight:
170.09
Beilstein:
2087388
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.341 (lit.)
n/D (lit.)
density
1.283 g/mL at 25 °C (lit.)
functional group
ester
fluoro
ketone
SMILES string
CCOC(=O)C(=O)C(F)(F)F
InChI
1S/C5H5F3O3/c1-2-11-4(10)3(9)5(6,7)8/h2H2,1H3
InChI key
KJHQVUNUOIEYSV-UHFFFAOYSA-N
General description
Ethyl 3,3,3-trifluoropyruvate is a trifluoromethylated compound. Enantioselective Friedel–Crafts alkylation of simple phenols and indoles with ethyl 3,3,3-trifluoropyruvate under different reaction conditions have been reported.
Application
Ethyl 3,3,3-trifluoropyruvate may be used in the preparation of N-heteroaryl(trifluoromethyl)hydroxyalkanoic acid esters.
accessory
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
87.8 °F - closed cup
Flash Point(C)
31 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of N-Heteroaryl (trifluoromethyl) hydroxyalkanoic Acid Esters by Highly Efficient Solid Acid-Catalyzed Hydroxyalkylation of Indoles and Pyrroles with Activated Trifluoromethyl Ketones.
Abid M and Torok B.
Advanced Synthesis & Catalysis, 347(14), 1797-1803 (2005)
Organocatalytic enantioselective Friedel?Crafts alkylation of simple phenols with trifluoropyruvate.
Zhao JL, et al.
Tetrahedron Letters, 49(9), 1476-1479 (2008)
Novel Enantiocomplementary C2-Symmetric Chiral Bis (imidazoline) Ligands: Highly Enantioselective Friedel?Crafts Alkylation of Indoles with Ethyl 3, 3, 3-Trifluoropyruvate.
Nakamura S, et al.
Advanced Synthesis & Catalysis, 350(10), 1443-1448 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service