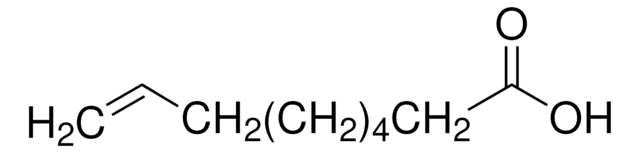All Photos(1)
About This Item
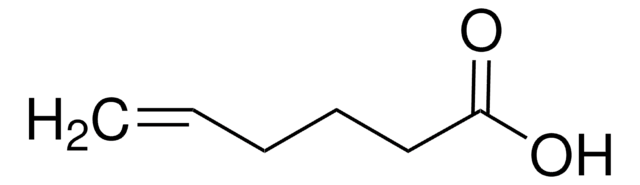
Linear Formula:
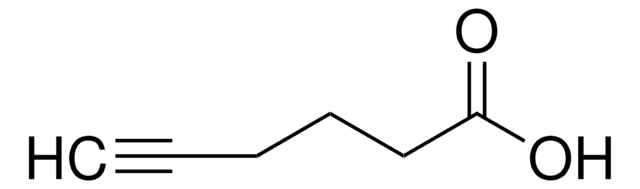
HC≡C(CH2)4COOH
CAS Number:
Molecular Weight:
126.15
Beilstein:
1747024
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
90%
refractive index
n20/D 1.451 (lit.)
bp
93-94 °C/1 mmHg (lit.)
density
0.997 g/mL at 25 °C (lit.)
SMILES string
OC(=O)CCCCC#C
InChI
1S/C7H10O2/c1-2-3-4-5-6-7(8)9/h1H,3-6H2,(H,8,9)
InChI key
OFCPMJGTZUVUSM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6-Heptynoic acid is an alkynoic acid with an acetylene bond. It undergoes condensation with various pyrroles to afford optical diverse fluorescent dyes with a terminal alkyne.
Application
6-Heptynoic acid may be used for the following syntheses:
- alkyne functionalized Boradiazaindacenes (BODIPY)dyes
- natural products epothilone B and D
- hymenialdisine (HMD) and aldisine (AD) affinity resins
- alkynyl esters
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A novel catalyst with a cuboidal PdMo3S4 core for the cyclization of alkynoic acids to enol lactones.
Wakabayashi T, et al.
Angewandte Chemie (International Edition in English), 35(18), 2123-2124 (1996)
R E Taylor et al.
Organic letters, 3(14), 2221-2224 (2001-07-07)
[reaction: see text] A highly convergent total synthesis of the natural products epothilone B and D is described. The route is highlighted by efficient generation of a C12-C13 trisubstituted olefin which exploits a sequential Nozaki-Hiyama-Kishi coupling and a stereoselective thionyl
Mariano Walter Pertino et al.
Molecules (Basel, Switzerland), 19(2), 2523-2535 (2014-02-26)
Dehydroabietic acid (DHA) is a naturally occurring diterpene with different and relevant biological activities. Previous studies have shown that some DHA derivatives display antiproliferative activity. However, the reported compounds did not include triazole derivatives. Starting from DHA (8,11,13-abietatrien-18-oic acid), and
Martijn Verdoes et al.
Bioorganic & medicinal chemistry letters, 17(22), 6169-6171 (2007-09-25)
The synthesis of three acetylene functionalized BODIPY dyes is described. These dyes are used to fluorescently modify an azido functionalized epoxomicin analogue employing the Huisgen 1,3-dipolar cycloaddition, resulting in a panel of fluorescent epoxomicin derived proteasome probes.
Lauren Ray et al.
Nature communications, 7, 13609-13609 (2016-12-22)
Type I modular polyketide synthases assemble diverse bioactive natural products. Such multienzymes typically use malonyl and methylmalonyl-CoA building blocks for polyketide chain assembly. However, in several cases more exotic alkylmalonyl-CoA extender units are also known to be incorporated. In all
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service