395439
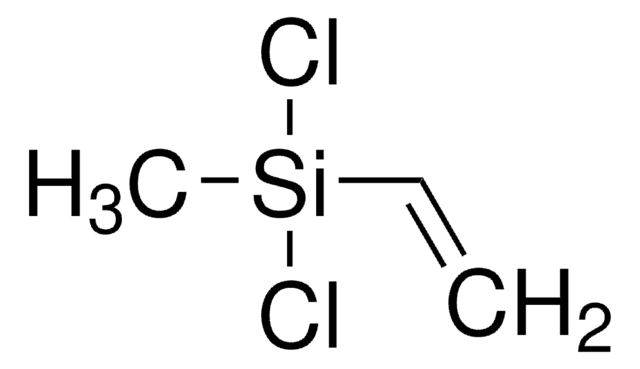
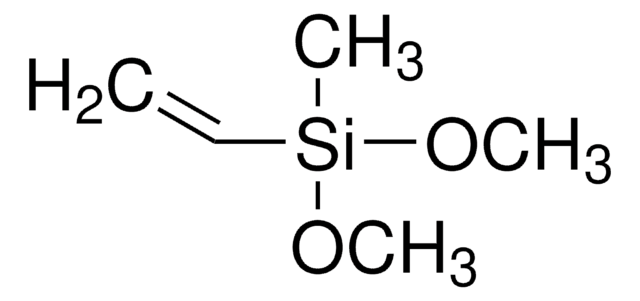
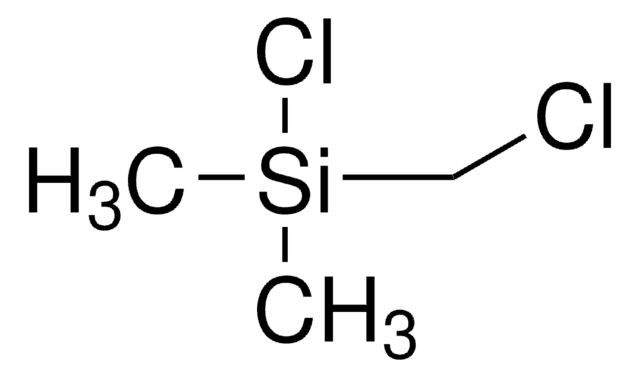
Chloro(dimethyl)vinylsilane
97%
Synonym(s):
Dimethylvinylchlorosilane, Vinyldimethylchlorosilane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2C=CHSi(CH3)2Cl
CAS Number:
Molecular Weight:
120.65
Beilstein:
1737688
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.414 (lit.)
bp
82-83 °C (lit.)
density
0.874 g/mL at 25 °C (lit.)
SMILES string
C[Si](C)(Cl)C=C
InChI
1S/C4H9ClSi/c1-4-6(2,3)5/h4H,1H2,2-3H3
InChI key
XSDCTSITJJJDPY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Chloro(dimethyl)vinylsilane (Dimethylvinylchlorosilane, Vinyldimethylchlorosilane, C4H9ClSi) is an organosilicon compound. It participates in the preparation of 1,1,2,2-tetramethyl-1,2-divinyldisilane. It undergoes [2+4] cycloaddition reaction with t-butyllithium in the presence of 2,3-dimethyl-1,3-butadiene to afford cycloadducts.
Application
Chloro(dimethyl)vinylsilane may be used in the synthesis of dimethyl(prop-2-ynyloxy)(vinyl)silane.
Chloro(dimethyl)vinylsilane may be used to prepare silicon-containing polymers, silaheterocycles, and new chelating ligands.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
23.0 °F - closed cup
Flash Point(C)
-5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Vinylmetalloids: I. Coupling reactions of group IV metalloidal halides with magnesium in tetrahydrofuran.
Soderquist JA and Hassner A
Journal of Organometallic Chemistry, 156(1), 227-233 (1978)
Zeitschrift fur Anorganische und Allgemeine Chemie, 608, 43-43 (1992)
Karolina Rachuta et al.
Physical chemistry chemical physics : PCCP, 21(36), 20384-20392 (2019-09-10)
In the course of studying silicon modifications to improve emission properties of commonly used organic compounds, biphenyl with dimethylsilylvinyl groups in the para position (3-Si) was investigated. A comparative study was performed on the exact C-analogue (3-C) and expanded to
The Journal of Organic Chemistry, 57, 5279-5279 (1992)
Schertzer BM.
The Development of Tandem Reactions Involving Enyne Metathesis to Form Substituted Cyclic Dienes, 209-209 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service