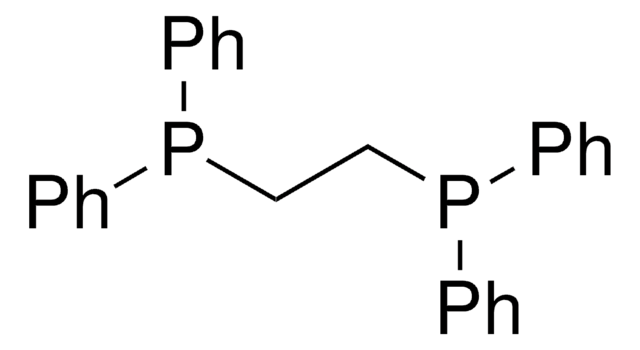
376728
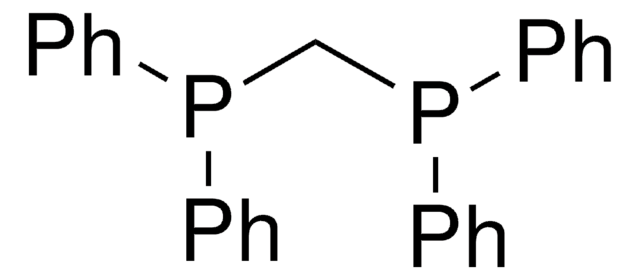
Ethylenebis(diphenylphosphine)
99%
Synonym(s):
1,2-Bis(diphenylphosphino)ethane, Diphos, dppe
About This Item
Recommended Products
Quality Level
Assay
99%
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
mp
137-142 °C (lit.)
functional group
phosphine
SMILES string
C(CP(c1ccccc1)c2ccccc2)P(c3ccccc3)c4ccccc4
InChI
1S/C26H24P2/c1-5-13-23(14-6-1)27(24-15-7-2-8-16-24)21-22-28(25-17-9-3-10-18-25)26-19-11-4-12-20-26/h1-20H,21-22H2
InChI key
QFMZQPDHXULLKC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 376728-5G | 4061831835274 |
| 376728-1G | 4061831835267 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service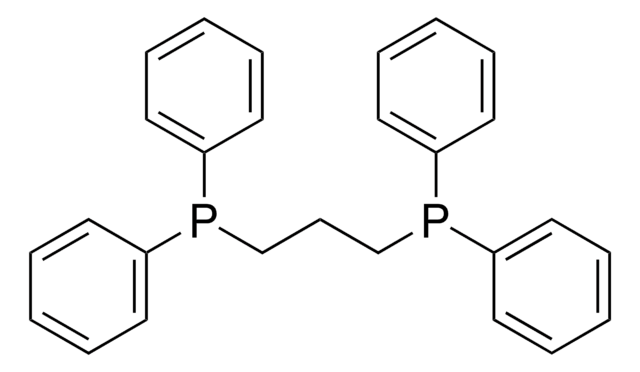
![[1,2-Bis(diphenylphosphino)ethane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/707/956/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf/640/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf.png)