All Photos(1)
About This Item
Empirical Formula (Hill Notation):
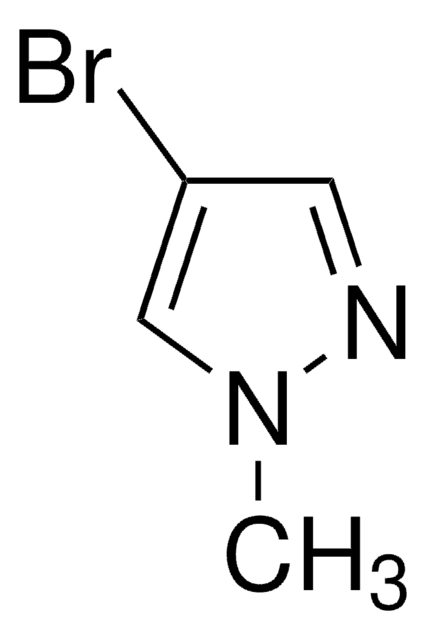
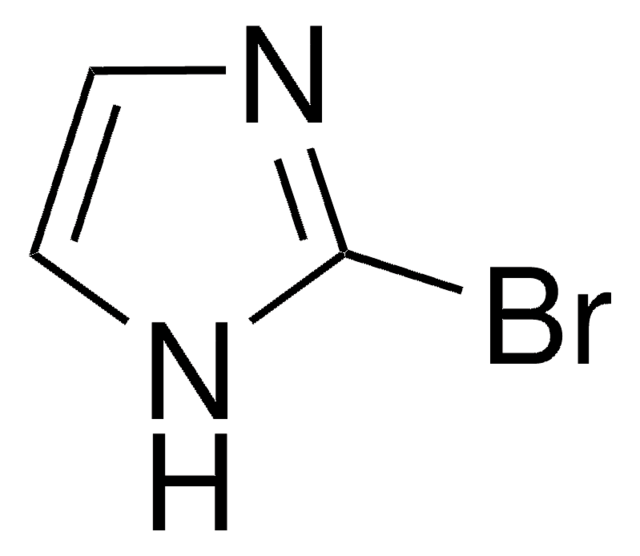
C3H3BrN2
CAS Number:
Molecular Weight:
146.97
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
250-260 °C (lit.)
mp
93-96 °C (lit.)
functional group
bromo
SMILES string
Brc1cn[nH]c1
InChI
1S/C3H3BrN2/c4-3-1-5-6-2-3/h1-2H,(H,5,6)
InChI key
WVGCPEDBFHEHEZ-UHFFFAOYSA-N
General description
4-Bromopyrazole is a heteroaryl halide and its cyanation in the presence of palladium catalysts has been reported.
4-Bromopyrazole is a pyrazole derivative. It is reported to react with titanium tetrachloride to afford binary adducts. Mutagenicity of 4-bromopyrazole has been tested using the L-arabinose forward mutation assay of Salmonella typhimurium. It is reported to inhibit the oxidative phosphorylation, the ATP-32P exchange reaction, and energy dependent and independent calcium uptake.
Application
4-Bromopyrazole may be used in the preparation of 4-bromo-1-(2-chloroethyl)-1H-pyrazole. It may be used as starting material in the synthesis of 1,4′-bipyrazoles.
4-Bromopyrazole may be used in the preparation of solid hexacoordinate complexes by reaction with dimethyl- and divinyl-tindichloride.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E Alejandre-Durán et al.
Environmental mutagenesis, 8(4), 611-619 (1986-01-01)
The mutagenicity of pyrazole and seven pyrazole derivatives (4-nitropyrazole, 4-bromopyrazole, 1-methyl-4-nitropyrazole, 3,5-dimethyl-4-nitropyrazole, 1-methyl-4-bromopyrazole, 4,4'-dinitro-1, 1'-methylene-dipyrazole and 4,4'-dibromo-1,1'-methylene-dipyrazole) has been investigated with the L-arabinose forward mutation assay of Salmonella typhimurium. Two nitroimidazoles (1-methyl-5-nitroimidazole and metronidazole) were included as reference drugs. The
Inhibition of the oxidation of the urinary bladder carcinogen N-butyl-N-(4-hydroxybutyl)nitrosamine by pyrazole and 4-substituted pyrazoles.
C C Irving et al.
Biochemical pharmacology, 37(8), 1642-1644 (1988-04-15)
Tetrahedron, 63, 748-748 (2007)
Dichlorodialkyltin complexes with 4-bromopyrazole. The crystal structure of bis(4-bromopyrazole- N2)dichlorodimethyltin(IV).
Casellato U, et al.
Journal of Organometallic Chemistry, 486(1-2), 105-107 (1995)
Ilia A. Guzei et al.
Inorganic chemistry, 36(20), 4415-4420 (2001-10-24)
Treatment of titanium tetrachloride with 3,5-di-tert-butylpyrazole affords the complexes [3,5-(C(CH(3))(3))(2)C(3)H(3)N(2)](2)[TiCl(6)] and (3,5-(C(CH(3))(3))(2)C(3)HN(2))(2)TiCl(2) in 37 and 42% yields, respectively. An analogous reaction with 3,5-dimethylpyrazole, 3-methylpyrazole, 4-bromopyrazole, and 4-iodopyrazole leads to the formation of corresponding TiCl(4)L(2) binary adducts in 30-86% yields. Crystal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service