369047
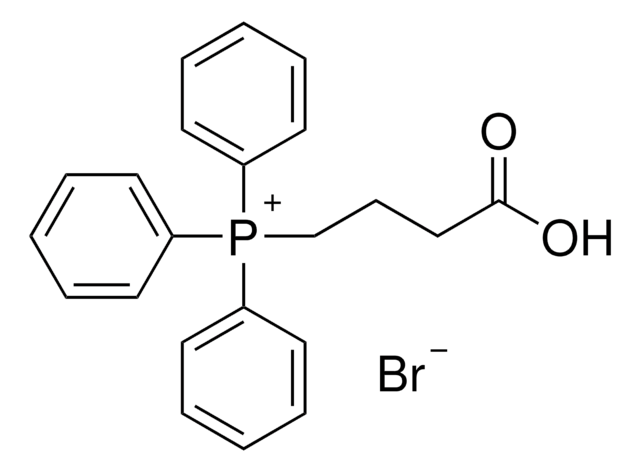
(tert-Butoxycarbonylmethyl)triphenylphosphonium bromide
98%
Synonym(s):
NSC 82468
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)3CO2CCH2P(C6H5)3Br
CAS Number:
Molecular Weight:
459.36
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
reaction suitability
reaction type: C-C Bond Formation
mp
178 °C (dec.) (lit.)
functional group
ester
phosphine
SMILES string
[Br-].CC(C)(C)OC(=O)C[P+](c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C24H26O2P.BrH/c1-24(2,3)26-23(25)19-27(20-13-7-4-8-14-20,21-15-9-5-10-16-21)22-17-11-6-12-18-22;/h4-18H,19H2,1-3H3;1H/q+1;/p-1
InChI key
ZGLFRTJDWWKIAK-UHFFFAOYSA-M
Application
Reactant for:
- Rhodium-catalyzed asymmetric hydrogenation reactions
- Wittig reaction
- Wittig methylenation
- Preparation of N-(phenylmethyl)-cis-pyrrolidinediacetic acid esters via double aza-Michael addition reaction
- Stereoselective preparation of of α-fluoro-α,β-unsaturated esters via deprotonation, followed by fluorination and stereoselective Wittig olefination with aldehydes
- Wittig chain extension reactions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service