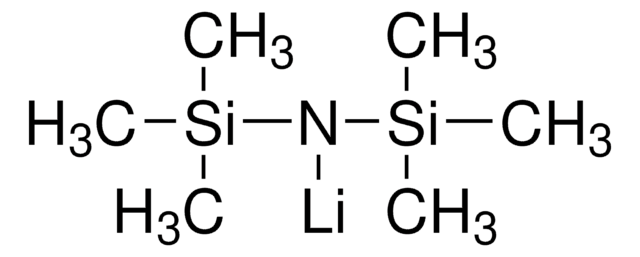324620
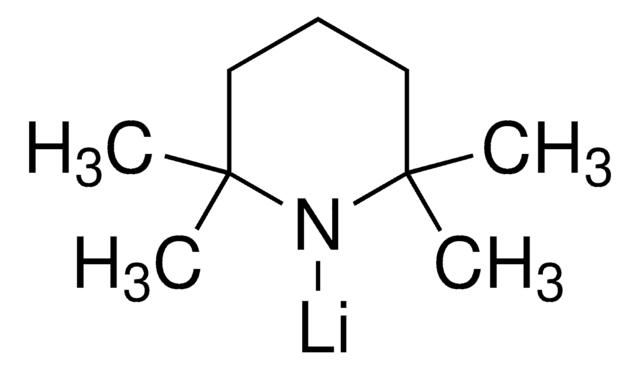
Lithium bis(trimethylsilyl)amide
97%
Synonym(s):
Hexamethyldisilazane lithium salt
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
[(CH3)3Si]2NLi
CAS Number:
Molecular Weight:
167.33
EC Number:
MDL number:
UNSPSC Code:
12352111
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
density
0.860 g/mL at 25 °C (lit.)
SMILES string
[Li]N([Si](C)(C)C)[Si](C)(C)C
InChI
1S/C6H18NSi2.Li/c1-8(2,3)7-9(4,5)6;/h1-6H3;/q-1;+1
InChI key
YNESATAKKCNGOF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Lithium bis(trimethylsilyl)amide is a non-nucleophilic strong Brønsted base, which is generally soluble in most of the nonpolar organic solvents. It is most commonly employed in organic reactions.
Application
Base employed in generating enolates for the preparation of lactone precursors, pyranones, and cyclohexanes.
Used to catalyze the addition of phosphine P-H bonds to carbodiimides leading to phosphaguanidines. Also used in a novel three-step synthesis of disubstituted 1,2,5-thiadiazoles.
Used to catalyze the addition of phosphine P-H bonds to carbodiimides leading to phosphaguanidines. Also used in a novel three-step synthesis of disubstituted 1,2,5-thiadiazoles.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Sol. 1 - Self-heat. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 2
Flash Point(F)
62.6 °F - closed cup
Flash Point(C)
17 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synlett, 507-507 (1993)
Tetrahedron Letters, 47, 8285-8285 (2006)
Wen-Xiong Zhang et al.
Chemical communications (Cambridge, England), (36), 3812-3814 (2006-09-14)
Organo alkali metal compounds such as (n)BuLi and (Me3Si)2NK act as excellent catalyst precursors for the addition of phosphine P-H bonds to carbodiimides, offering a general and atom-economical route to substituted phosphaguanidines, with excellent tolerability to aromatic C-Br and C-Cl
The Journal of Organic Chemistry, 58, 7304-7304 (1993)
Tetrahedron, 50, 9061-9061 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service