317047
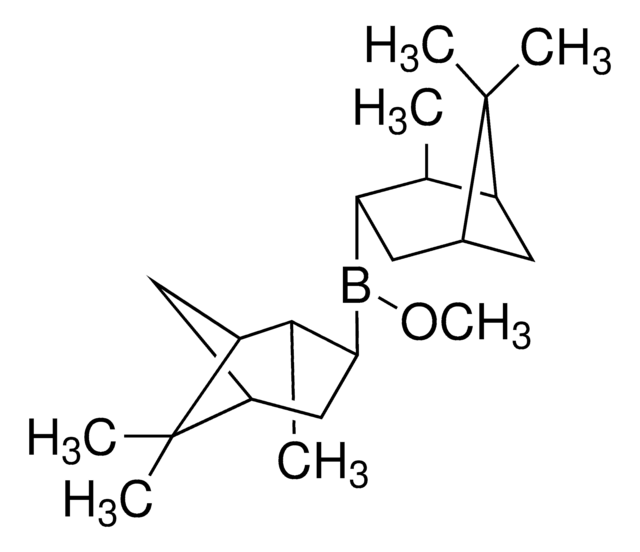
(−)-B-Methoxydiisopinocampheylborane
Synonym(s):
(−)-Diisopinocampheylmethoxyborane
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C21H37BO
CAS Number:
Molecular Weight:
316.33
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
SMILES string
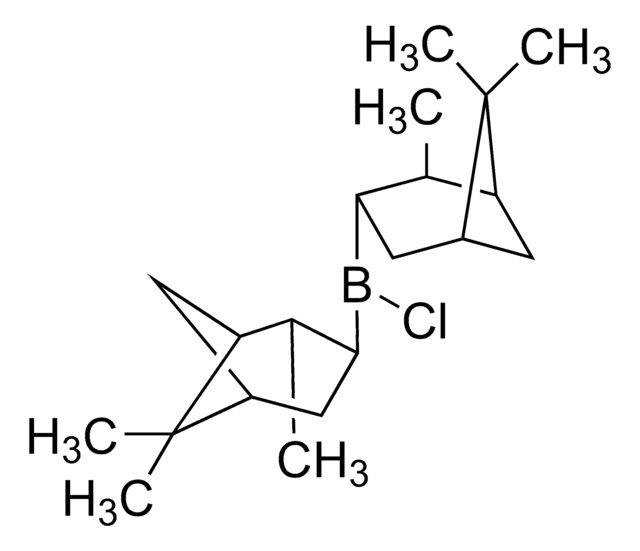
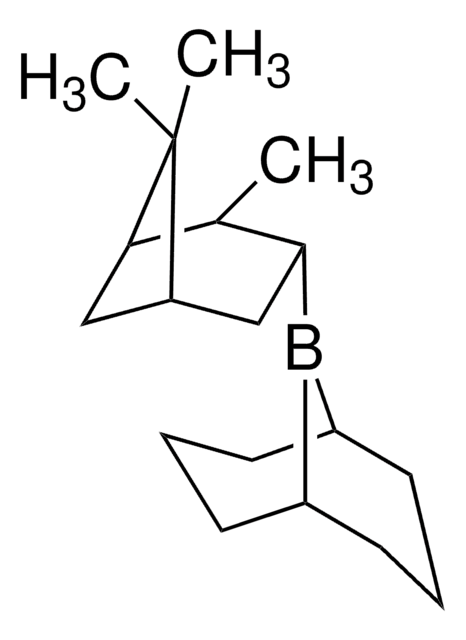
COB([C@H]1CC2CC([C@@H]1C)C2(C)C)[C@H]3CC4CC([C@@H]3C)C4(C)C
InChI
1S/C21H37BO/c1-12-16-8-14(20(16,3)4)10-18(12)22(23-7)19-11-15-9-17(13(19)2)21(15,5)6/h12-19H,8-11H2,1-7H3/t12-,13-,14-,15-,16+,17+,18-,19-/m0/s1
InChI key
IAQXEQYLQNNXJC-BAMGFKBFSA-N
General description
B-Methoxydiisopinocampheylborane (Ipc2BOMe) is an organoborane compound, which is prepared from excess α-pinene, borane dimethylsulfide, and methanol via the formation of an intermediate diisocampheylborane. Ipc2BOMe is used as a versatile reagent for the construction of C-C bonds in asymmetric synthesis.
Application
Reactant involved in organic synthesis reactions such as:
- Double allylboration for synthesis of fragments of tetrafibricin
- Anticancer cytotoxic monorhizopodin synthesis
- Annulation of cyclic allylsilanes
- Asymmetric synthesis of β-amino-α-hydroxy acid taxol side chain analogs
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service