268100
Tetrabutylammonium phosphate monobasic solution
1.0 M in H2O
Synonym(s):
Tetrabutylammonium dihydrogen phosphate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
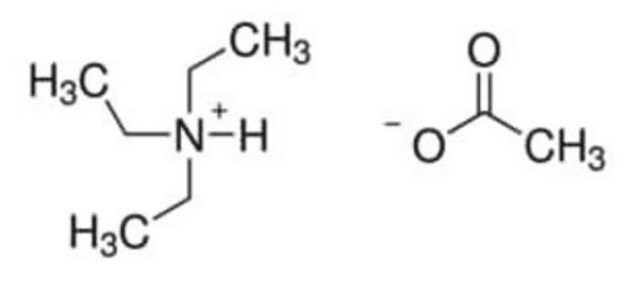
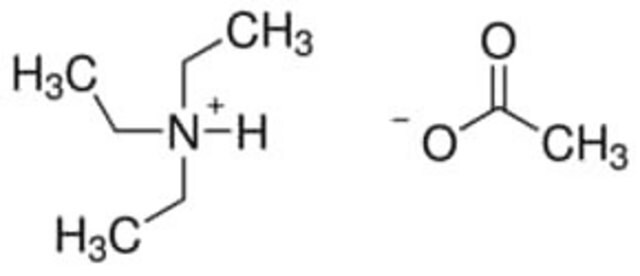
(CH3CH2CH2CH2)4N[OP(OH)2O]
CAS Number:
Molecular Weight:
339.45
Beilstein:
5196532
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
concentration
1.0 M in H2O
density
1.037 g/mL at 25 °C
SMILES string
OP(O)([O-])=O.CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.H3O4P/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;1-5(2,3)4/h5-16H2,1-4H3;(H3,1,2,3,4)/q+1;/p-1
InChI key
ARRNBPCNZJXHRJ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Tetrabutylammonium phosphate monobasic solution (TABP) is a quaternary ammonium salt commonly used in the preparation of buffers and ion-pairing reagents.
Application
Tetrabutylammonium phosphate monobasic solution can be used as a:
- Catalyst for the selective N-alkylation of indoles with electron-poor alkenes.
- Hydrogen-bond-acceptor (HBA) catalyst to synthesize ketoesters via αC-H bond activation.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron, 62, 10237-10237 (2006)
Journal of Liquid Chromatography, 15, 2487-2487 (1992)
Journal of Chromatographic Science, 31, 231-231 (1993)
R Bryan Sears et al.
Journal of inorganic biochemistry, 121, 77-87 (2013-01-29)
The complex cis-[Ru(phpy)(phen)(CH3CN)2](+) (phpy=2-phenylpyridine, phen=1,10-phenanthroline) was investigated as a potential photodynamic therapy (PDT) agent. This complex presents desirable photochemical characteristics including a low energy absorption tail extending into the PDT window (600-850nm) and photoinduced exchange of the CH3CN ligands, generating
Oliver Bixner et al.
The Journal of chemical physics, 136(20), 204503-204503 (2012-06-07)
The interaction of exciton and charge transfer (CT) states plays a central role in photo-induced CT processes in chemistry, biology, and physics. In this work, we use a combination of two-dimensional electronic spectroscopy (2D-ES), pump-probe measurements, and quantum chemistry to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service