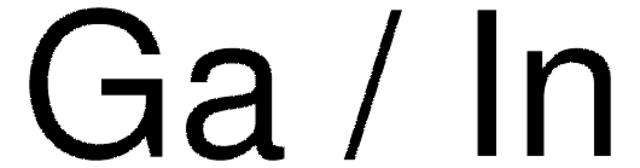263265
Gallium
99.99% trace metals basis
Synonym(s):
Elemental gallium
About This Item
Recommended Products
Assay
99.99% trace metals basis
form
(metallic liquid or solid (ambient temp dependent))
resistivity
25.795 μΩ-cm, 30°C
bp
2403 °C (lit.)
mp
29.8 °C (lit.)
density
5.904 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
[Ga]
InChI
1S/Ga
InChI key
GYHNNYVSQQEPJS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Gallium nitride and other Ga-based compounds for optoelectronic applications.
- Sulfur/gallium hybrid cathode material for lithium-sulfur batteries. The surface of Ga metal promotes the redox reaction of lithium polysulfides due to its electrocatalytic effect.
- Ga-based liquid alloys for the fabrication offlexible electronic devices.
Features and Benefits
- Excellent conductor of heat and electricity
- Wide temperature range in the liquid state
- Lowvapor pressure at high temperature
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Met. Corr. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Solid state and materials chemistry have made a tremendous impact and have experienced growth in recent years, particularly for rare earthcontaining materials.
Technologies are an integral part of our lives and we rely on them for such things as communication, heating and cooling, transportation, and construction. Improvements to technologies have made what they do for us more precise, automated, efficient, and powerful.
The unique properties of the rare-earth elements and their alloys have brought them from relative obscurity to high profile use in common hightech applications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service