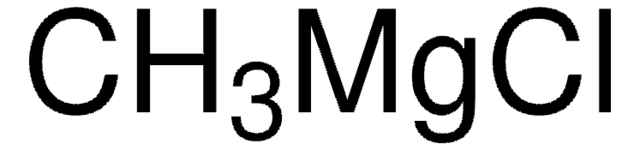254363
Methylmagnesium iodide solution
3.0 M in diethyl ether
Synonym(s):
Iodomethylmagnesium, Methylmagnesium iodine
About This Item
Recommended Products
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
3.0 M in diethyl ether
density
1.261 g/mL at 25 °C
SMILES string
C[Mg]I
InChI
1S/CH3.HI.Mg/h1H3;1H;/q;;+1/p-1
InChI key
AUPXBVDHVRZMIB-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
- To prepare cis-1,2-dialkylcyclopropanols by treating with titanium(IV) alkoxide and carboxylic esters.
- In stereospecific Ni catalyzed Kumada cross-coupling reactions of benzylic ethers.
- To prepare 2,4-disubstituted selenochromenes by reacting with 1-benzoselenopyrylium salts.
- In one of the key synthetic steps for the preparation of 7β-methyl-substituted 5-androstene derivatives from 3β-acetoxyandrost-5-en-17-one stereoselectively.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 1
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
-40.0 °F - closed cup
Flash Point(C)
-40 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service