All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H10N2
CAS Number:
Molecular Weight:
158.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
solid
mp
96-98 °C (lit.)
SMILES string
Nc1ccccc1-n2cccc2
InChI
1S/C10H10N2/c11-9-5-1-2-6-10(9)12-7-3-4-8-12/h1-8H,11H2
InChI key
GDMZHPUPLWQIBD-UHFFFAOYSA-N
Related Categories
General description
1-(2-Aminophenyl)pyrrole participates in Pt(IV)-catalyzed hydroamination triggered cyclization reaction to yield fused pyrrolo [1,2-a] quinoxalines. It reacts with aromatic or heteroaromatic aldehydes in ethanol and catalytic amounts of acetic acid to yield 4,5-dihydropyrrolo[1,2-a]quinoxalines. Thin films of poly(1-(2-aminophenyl)pyrrole) has been prepared via oxidative electropolymerization.
Application
1-(2-Aminophenyl)pyrrole was used in the synthesis of 4-substituted pyrrolo[1,2-a]quinoxaline derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nitin T Patil et al.
The Journal of organic chemistry, 75(10), 3371-3380 (2010-04-16)
A PtCl(4)-catalyzed hydroamination-triggered cyclization strategy to access biologically interesting N-containing heterocycles such as pyrrolo[1,2-a]quinoxalines, indolo[1,2-a]quinoxalines, and indolo[3,2-c]quinolines is described. The reaction makes use of aminoaromatics such as 1-(2-aminophenyl)pyrroles, N-(2-aminophenyl)indoles, 2-(2-aminophenyl)indoles, and alkynes having a tethered hydroxyl group. Mechanistically, the reaction
Jean Guillon et al.
Bioorganic & medicinal chemistry, 15(1), 194-210 (2006-10-20)
An original series of 4-substituted pyrrolo[1,2-a]quinoxaline derivatives, new structural analogues of Galipea species quinoline alkaloids, was synthesized from various substituted 2-nitroanilines via multistep heterocyclizations and tested for in vitro antiparasitic activity upon Leishmania amazonensis and Leishmania infantum strains. Structure-activity relationships
Photoelectrochemical studies on poly [1-(2-aminophenyl) pyrrole]-Creation of a photoactive inorganic-organic semiconductor interface (IOI).
Kasem KK, et al.
Canadian Journal of Chemistry, 87(8), 1109-1116 (2009)
A Versatile Synthesis of 4, 5-Dihydropyrrolo [1, 2-a] quinoxalines.
Abonia R, et al.
Journal of Heterocyclic Chemistry, 38(3), 671-674 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service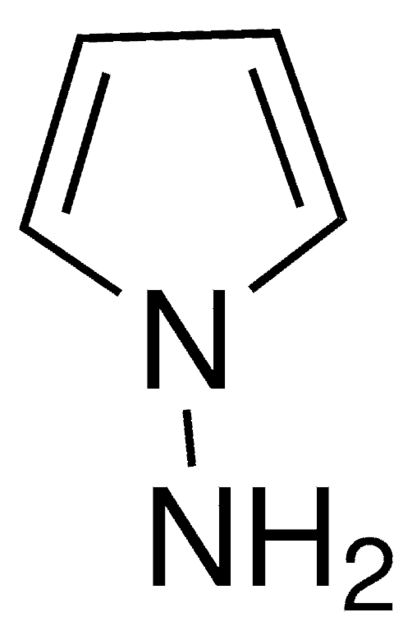

![Potassium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate Selectophore™](/deepweb/assets/sigmaaldrich/product/structures/631/130/b5486f44-2e69-40d0-902f-dd71894a6add/640/b5486f44-2e69-40d0-902f-dd71894a6add.png)