All Photos(1)
About This Item
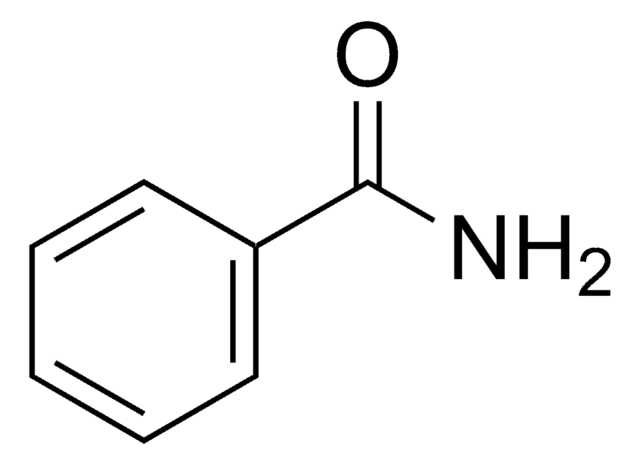
Linear Formula:
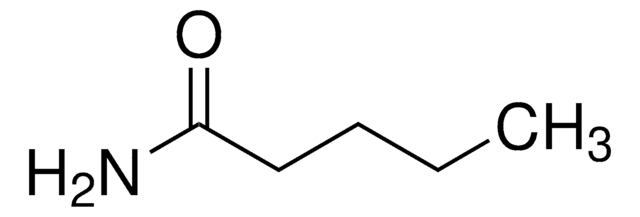
CH3CH2CH2CONH2
CAS Number:
Molecular Weight:
87.12
Beilstein:
1361528
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Assay:
≥98.0% (T)
Recommended Products
Quality Level
Assay
≥98.0% (T)
mp
114-116 °C
solubility
alcohol: soluble(lit.)
diethyl ether: slightly soluble(lit.)
water: soluble(lit.)
functional group
amide
SMILES string
CCCC(N)=O
InChI
1S/C4H9NO/c1-2-3-4(5)6/h2-3H2,1H3,(H2,5,6)
InChI key
DNSISZSEWVHGLH-UHFFFAOYSA-N
Gene Information
rat ... Ggt1(116568)
Looking for similar products? Visit Product Comparison Guide
Application
Butyramide was used in the synthesis of hydroxamic acids, electrorheological fluids and β-amodoorganotin compounds. It was used as substrate of (+)-γ-lactamase to develop a microreactor to study enzyme stability, activity, kinetics and substrate specificity.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hongjian Zhang et al.
Drug metabolism and disposition: the biological fate of chemicals, 35(5), 795-805 (2007-02-17)
2-{Butyryl-[2'-(4,5-dimethyl-isoxazol-3-ylsulfamoyl)-biphenyl-4-ylmethyl]-amino}-N-isopropyl-3-methyl-butyramide (BMS-1) is a potent dual acting angiotensin-1 and endothelin-A receptor antagonist. The compound was subject to rapid metabolic clearance in monkey and human liver microsomes and exhibited low systemic exposure and marked interanimal variability in cynomolgus monkeys after p.o.
Effect of butyrate analogues on proliferation and differentiation in human neuroblastoma cell lines.
P Rocchi et al.
Anticancer research, 18(2A), 1099-1103 (1998-06-06)
Butyric acid has been shown in vitro to produce cytodifferentiation of a wide variety of neoplastic cells. The potential clinical use of this compound as a therapeutic agent is limited by its rapid metabolism. This has led to the examination
B P O'Hara et al.
Protein engineering, 13(2), 129-132 (2000-03-10)
The AmiC protein in Pseudomonas aeruginosa is the negative regulator and ligand receptor for an amide-inducible aliphatic amidase operon. In the wild-type PAC1 strain, amidase expression is induced by acetamide or lactamide, but not by butyramide. A mutant strain of
Rajendra Singh et al.
Bioprocess and biosystems engineering, 41(8), 1225-1232 (2018-05-12)
Butyramide is a commodity chemical having wide range of applications from material science to biological sciences including synthesis of therapeutic drugs, hydroxamic acids, and electrorheological fluids. The nitrile hydratase protein of Bacillus sp. APB-6 was explored to develop an efficient
R A Norman et al.
The Journal of biological chemistry, 275(39), 30660-30667 (2000-07-13)
Expression of the amidase operon of Pseudomonas aeruginosa is controlled by AmiC, the ligand sensor and negative regulator, and AmiR the transcription antitermination factor activator. We have titrated out AmiC repression activity in vivo by increased AmiR production in trans
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service