184055
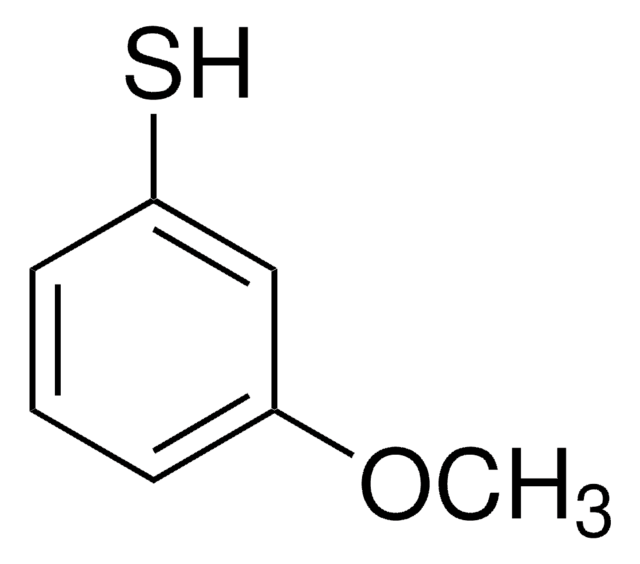
2-Methoxythiophenol
97%
Synonym(s):
2-Mercaptoanisole, 2-Methoxybenzenethiol, Thioguaiacol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H4SH
CAS Number:
Molecular Weight:
140.20
Beilstein:
2042178
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.591 (lit.)
bp
99 °C/8 mmHg (lit.)
density
1.152 g/mL at 25 °C (lit.)
SMILES string
COc1ccccc1S
InChI
1S/C7H8OS/c1-8-6-4-2-3-5-7(6)9/h2-5,9H,1H3
InChI key
DSCJETUEDFKYGN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Methoxythiophenol was used in the synthesis of:
- (4S)-N-[(2S)-2-methy-3-(2-methoxyphenylthio)propanoyl]-4-phenyloxazolidin-2-one
- 2-(4-fluorophenyl)-6-(2-methoxyphenylsulfanyl)imidazo[1,2-a]pyridine
- 2-(2-methoxyphenylsulfanyl)benzoic acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
2-(2-Methoxyphenylsulfanyl) benzoic acid.
Li A-J, et al.
Acta Crystallographica Section E, Structure Reports Online, 62(3), o1176-o1177 (2006)
A general and efficient method for the copper-catalyzed cross-coupling of amides and thiophenols with 6-halogenoimidazo [1, 2-a] pyridines.
Enguehard-Gueiffier C, et al.
Tetrahedron, 62(25), 6042-6049 (2006)
Asymmetric conjugate addition of thiols to chiral methacryloyloxazolidinones.
Tseng T-C and Wu M-J.
Tetrahedron Asymmetry, 6(7), 1633-1640 (1995)
Acute toxicity of thioguaiacol and of versalide in rodents.
K R Butterworth et al.
Food and cosmetics toxicology, 19(6), 753-755 (1981-12-01)
Jennifer A Jacobsen et al.
Journal of medicinal chemistry, 54(2), 591-602 (2010-12-30)
Fragment-based lead design (FBLD) has been used to identify new metal-binding groups for metalloenzyme inhibitors. When screened at 1 mM, a chelator fragment library (CFL-1.1) of 96 compounds produced hit rates ranging from 29% to 43% for five matrix metalloproteases
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service