158186
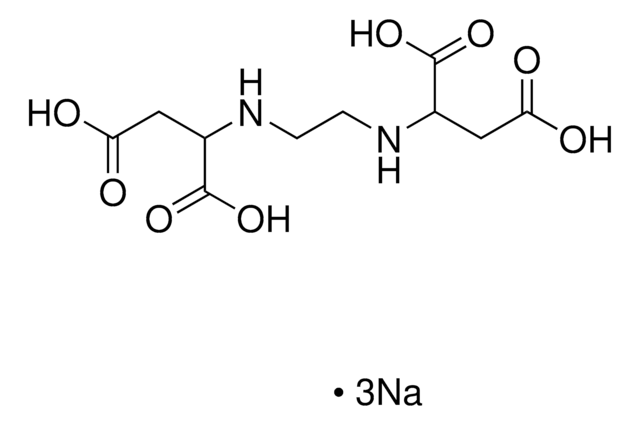
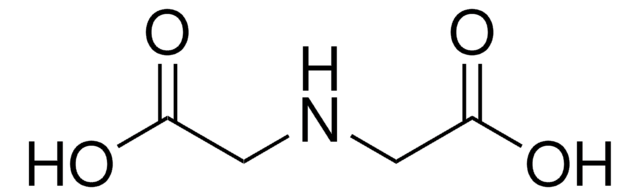
Ethylenediamine-N,N′-diacetic acid
≥98%
Synonym(s):
EDDA, N,N′-Ethylenediglycine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOOCCH2NHCH2CH2NHCH2COOH
CAS Number:
Molecular Weight:
176.17
Beilstein:
1778355
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
mp
224 °C (dec.) (lit.)
functional group
amine
carboxylic acid
SMILES string
OC(=O)CNCCNCC(O)=O
InChI
1S/C6H12N2O4/c9-5(10)3-7-1-2-8-4-6(11)12/h7-8H,1-4H2,(H,9,10)(H,11,12)
InChI key
IFQUWYZCAGRUJN-UHFFFAOYSA-N
Application
Ethylenediamine-N,N′-diacetic acid (EDDA) is a chelating agent that can be used to synthesize:
- Binary and ternary copper(II) complexes with potent proteasome inhibitory properties.
- Pd(EDDA) complexes which can coordinate with amino acids, peptides, or DNA units.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Clara L Santos-Cuevas et al.
Nuclear medicine communications, 29(8), 741-747 (2008-08-30)
The gastrin-releasing peptide receptor (GRP-R) is expressed in several normal human tissues and is overexpressed in various human tumors including breast, prostate, small-cell lung cancer and pancreatic cancer. Recently, 99mTc-EDDA/HYNIC-[Lys]-bombesin (99mTc-HYNIC-BN) was reported as a radiopharmaceutical with high stability in
Olivier Dalmas et al.
Structure (London, England : 1993), 18(7), 868-878 (2010-07-20)
The transmembrane conformation of Thermotoga maritima CorA, a magnesium transport system, has been studied in its Mg(2+)-bound form by site-directed spin labeling and electron paramagnetic resonance spectroscopy. Probe mobility together with accessibility data were used to evaluate the overall dynamics
Alma D Miranda-Olvera et al.
Bioconjugate chemistry, 18(5), 1560-1567 (2007-08-02)
Two synthetic procedures for HYNIC oxytocin labeling were developed: one based on an orthogonal protection approach and the other with prelabeled (Boc)HYNIC-(Fmoc) amino acids. Both procedures were compared and applied to the preparation of several HYNIC-oxytocin derivatives where ligand position
Jelena M Vujić et al.
European journal of medicinal chemistry, 45(9), 3601-3606 (2010-06-24)
Four novel bidentate N,N'-ligand precursors, including O,O'-dialkyl esters (alkyl = ethyl, n-propyl, n-butyl and n-pentyl), L1 x 2 HCl-L4 x 2 HCl, of (S,S)-ethylenediamine-N,N'-di-2-(4-methyl)-pentanoic acid dihydrochloride [(S,S)-H(4)eddl]Cl(2) and the corresponding palladium(II) complexes 1-4, were prepared and characterized by IR, (1)H
Levente K Meszaros et al.
Dalton transactions (Cambridge, England : 2003), 40(23), 6260-6267 (2011-02-26)
6-Hydrazinonicotinic acid (HYNIC, 1) is a well-established bifunctional technetium-binding ligand often used to synthesise bioconjugates for radiolabelling with Tc-99m. It is capable of efficient capture of technetium at extremely low concentrations, but the structure of the labelled complexes is heterogeneous
Global Trade Item Number
| SKU | GTIN |
|---|---|
| S892173-1EA | |
| 158186-1G | 4061838743794 |
| 158186-25G | |
| 158186-1KG | |
| 158186-5G | 4061838743800 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service