157422
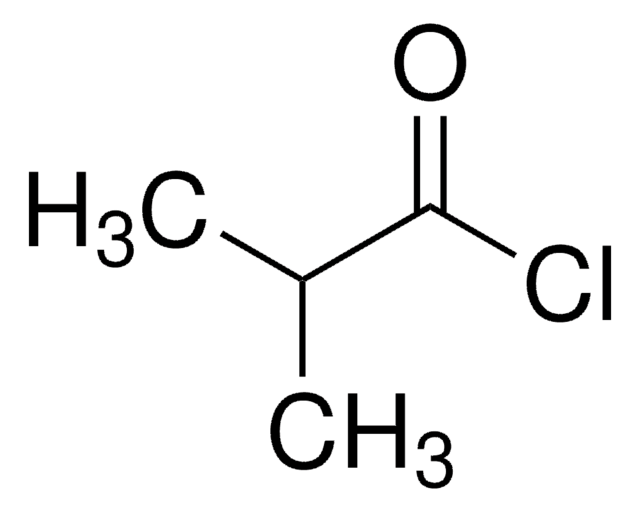
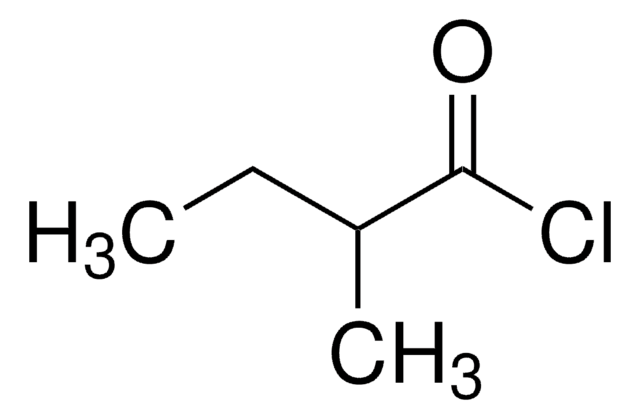
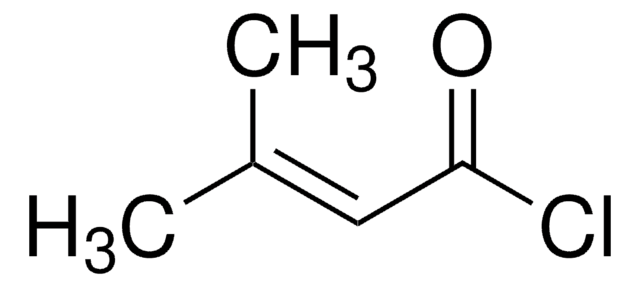
Isovaleryl chloride
98%
Synonym(s):
3-Methylbutyryl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH2COCl
CAS Number:
Molecular Weight:
120.58
Beilstein:
741910
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.416 (lit.)
bp
115-117 °C (lit.)
density
0.989 g/mL at 25 °C (lit.)
functional group
acyl chloride
SMILES string
CC(C)CC(Cl)=O
InChI
1S/C5H9ClO/c1-4(2)3-5(6)7/h4H,3H2,1-2H3
InChI key
ISULZYQDGYXDFW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Isovaleryl chloride was used in the synthesis of:
- furanodictines A and B
- tetrapeptide amide, S-benzyl-L-cysteinyl-L-prolyl-L-leucylglycinamide
- (+)-blastmycinone
- (R)- and (S)-2-methyl-4-octanol, aggregation pheromone of some sugarcane weevils
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
87.8 °F - closed cup
Flash Point(C)
31 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enantioselective synthesis of (R)-and (S)-2-methyl-4-octanol, the male-produced aggregation pheromone of Curculionidae species.
Baraldi PT, et al.
Tetrahedron Asymmetry, 13(6), 621-624 (2002)
The Synthesis of the Tetrapeptide Amide S-Benzyl-L-cysteinyl-L-prolyl-L-leucylglycinamide
Ressler Cand Vincent du Vigneaud.
Journal of the American Chemical Society, 76(12), 3107-3109 (1954)
Makoto Ogata et al.
Carbohydrate research, 345(2), 230-234 (2009-12-08)
A novel synthesis of furanodictines A [2-acetamido-3,6-anhydro-2-deoxy-5-O-isovaleryl-D-glucofuranose (1)] and B [2-acetamido-3,6-anhydro-2-deoxy-5-O-isovaleryl-D-mannofuranose (2)] is described starting from 2-acetamido-2-deoxy-D-glucose (GlcNAc). The synthetic protocol is based on deriving the epimeric bicyclic 3,6-anhydro sugars [2-acetamido-3,6-anhydro-2-deoxy-D-glucofuranose (4) and 2-acetamido-3,6-anhydro-2-deoxy-D-mannofuranose (5)] from GlcNAc. Reaction with borate
Organoaluminium induced ring-opening of epoxypyranosides. V. Formal total synthesis of antimycin A3 and synthesis of (+)-blastmycinone.
Inghardt T and Frejd T.
Tetrahedron, 47(32), 6483-6492 (1991)
Jessica L Wojtaszek et al.
Cell, 178(1), 152-159 (2019-06-11)
Intrinsic and acquired drug resistance and induction of secondary malignancies limit successful chemotherapy. Because mutagenic translesion synthesis (TLS) contributes to chemoresistance as well as treatment-induced mutations, targeting TLS is an attractive avenue for improving chemotherapeutics. However, development of small molecules with
Global Trade Item Number
| SKU | GTIN |
|---|---|
| S916536-1EA | |
| 157422-100G | 4061838743329 |
| 157422-25G | 4061838743336 |
| 157422-500G | 4061838743343 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service