135577
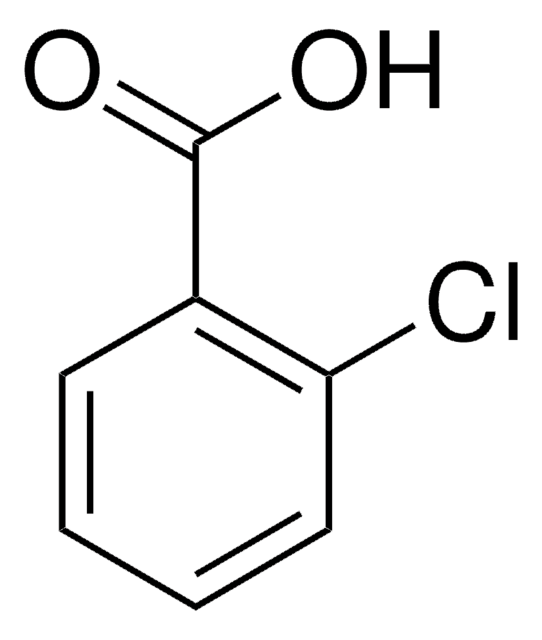
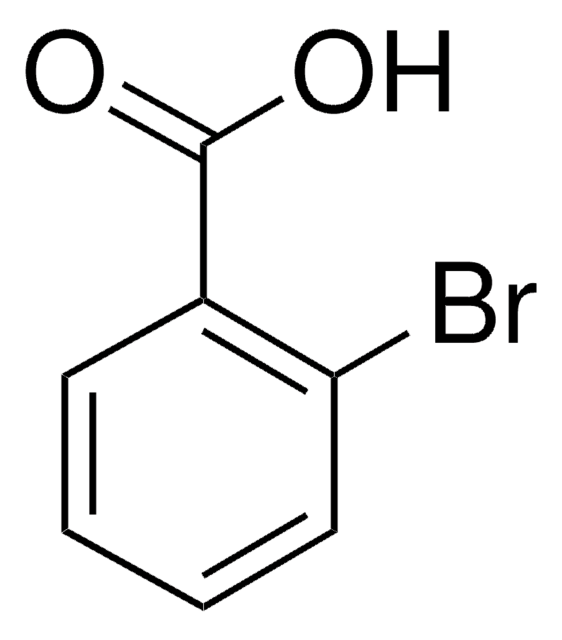
2-Chlorobenzoic acid
98%
Synonym(s):
2-CBA, o-Chlorobenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4CO2H
CAS Number:
Molecular Weight:
156.57
Beilstein:
907340
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
138-140 °C (lit.)
solubility
cold water: soluble 900 part
alcohol: freely soluble
diethyl ether: freely soluble
water: soluble (hot)
functional group
carboxylic acid
chloro
SMILES string
OC(=O)c1ccccc1Cl
InChI
1S/C7H5ClO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,(H,9,10)
InChI key
IKCLCGXPQILATA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Chlorobenzoic acid degradation by pure cultures of Burkholderia cepacia strain with and without the bacterial hemoglobin gene was studied in parallel membrane bioreactors. Intramolecular hydrogen atom tunneling in 2-chlorobenzoic acid has been studied by low-temperature matrix-isolation infrared spectroscopy.
Application
2-Chlorobenzoic acid was used to study the degradation of 2-bromobenzoic acid by Pseudomonas aeruginosa.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point(F)
343.4 °F
Flash Point(C)
173 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
F K Higson et al.
Applied and environmental microbiology, 56(6), 1615-1619 (1990-06-01)
A strain of Pseudomonas aeruginosa producing 2-bromobenzoic acid, designated 2-BBZA, was isolated by enrichment culture from municipal sewage. It degraded all four 2-halobenzoates as well as certain 3-halo- and dihalobenzoates, though none of the 4-halobenzoates supported growth of this organism.
W J Hickey et al.
Applied and environmental microbiology, 67(10), 4603-4609 (2001-09-26)
We have identified in Pseudomonas aeruginosa strain JB2 a novel cluster of mobile genes encoding degradation of hydroxy- and halo-aromatic compounds. Nineteen open reading frames were located and, based on sequence similarities, were putatively identified as encoding a ring hydroxylating
W J Hickey et al.
Applied and environmental microbiology, 67(12), 5648-5655 (2001-11-28)
Protein mass spectrometry and molecular cloning techniques were used to identify and characterize mobile o-halobenzoate oxygenase genes in Pseudomonas aeruginosa strain JB2 and Pseudomonas huttiensis strain D1. Proteins induced in strains JB2 and D1 by growth on 2-chlorobenzoate (2-CBa) were
Meltem Urgun-Demirtas et al.
Biotechnology and bioengineering, 87(1), 110-118 (2004-06-24)
Application of Vitreoscilla hemoglobin (VHb) technology to 2-CBA degradation by Burkholderia cepacia strain DNT under hypoxic conditions was studied in continuous culture chemostats. Dechlorination abilities of both recombinant (VHb gene (vgb) containing) and untransformed cells were investigated at various dilution
A S Yuroff et al.
Applied and environmental microbiology, 69(12), 7401-7408 (2003-12-09)
We investigated the mechanisms of uptake of 2-chlorobenzoate (2-CBa) and 2-hydroxybenzoate (2-HBa) by Pseudomonas huttiensis strain D1. Uptake was monitored by assaying intracellular accumulation of 2-[UL-ring-14C]CBa and 2-[UL-ring-14C]HBa. Uptake of 2-CBa showed substrate saturation kinetics with an apparent Km of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service