105902
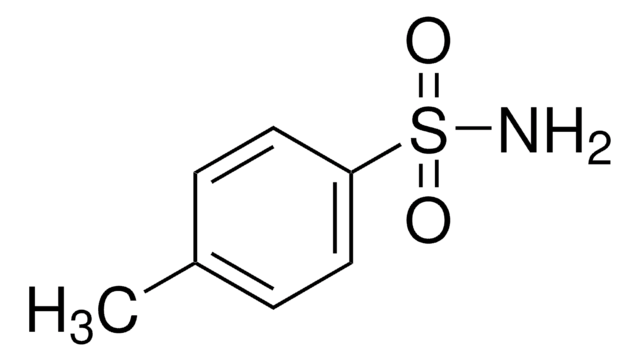
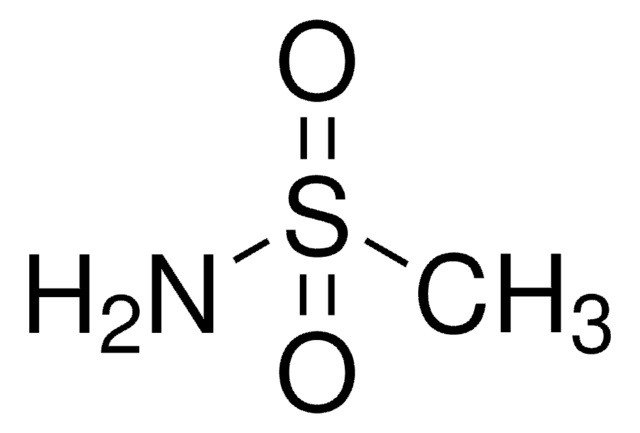
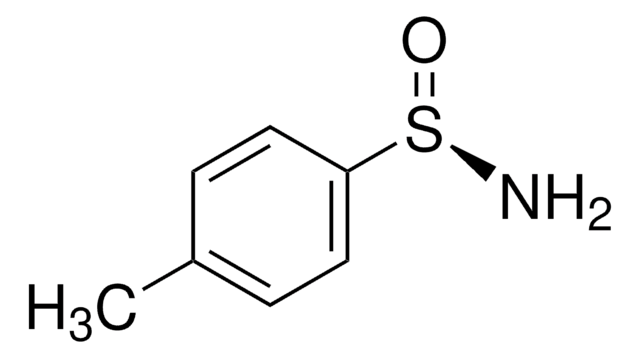
p-Toluenesulfonamide
reagent grade, 97%
Synonym(s):
4-Methylbenzene-1-sulfonamide, p-Tosylamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3C6H4SO2NH2
CAS Number:
Molecular Weight:
171.22
Beilstein:
472689
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
reagent grade
Quality Level
Assay
97%
form
solid
mp
134-137 °C (lit.)
solubility
DMSO: soluble
functional group
sulfonamide
SMILES string
Cc1ccc(cc1)S(N)(=O)=O
InChI
1S/C7H9NO2S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3,(H2,8,9,10)
InChI key
LMYRWZFENFIFIT-UHFFFAOYSA-N
Gene Information
human ... CA1(759) , CA2(760) , CA5A(763) , CA5B(11238)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
p-Toluenesulfonamide undergoes FeCl3-catalyzed direct substitution reaction with benzylic and allylic alcohols.It is employed as nucleophile in tetrabutylammonium fluoride (TBAF) catalyzed vinyl aziridine opening reaction.
Application
p-Toluenesulfonamide was used to prepare the precursor required for synthesis of ethyl 6-phenyl-1-tosyl-1,2,5,6-tetrahydropyridine-3-carboxylate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
395.6 °F - closed cup
Flash Point(C)
202 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
PHOSPHINE-CATALYZED [4+2] ANNULATION: SYNTHESIS OF ETHYL 6-PHENYL-1-TOSYL-1,2,5,6-TETRAHYDROPYRIDINE-3-CARBOXYLATE.
Kui Lu et al.
Organic syntheses; an annual publication of satisfactory methods for the preparation of organic chemicals, 2009(86), 212-224 (2010-02-18)
An efficient FeCl3 -catalyzed amidation reaction of secondary benzylic and allylic alcohols with carboxamides or p-toluenesulfonamide.
Jana U, et al.
Tetrahedron Letters, 42(37), 6433-6435 (2001)
Charles H Reynolds et al.
Journal of medicinal chemistry, 51(8), 2432-2438 (2008-04-03)
Ligand efficiency (i.e., potency/size) has emerged as an important metric in drug discovery. In general, smaller, more efficient ligands are believed to have improved prospects for good drug properties (e.g., bioavailability). Our analysis of thousands of ligands across a variety
Yang Yang et al.
Organic letters, 13(20), 5608-5611 (2011-09-16)
BF(3)·OEt(2)-catalyzed direct cyanation of indoles and pyrroles using a less toxic, bench-stable, and easily handled electrophilic cyanating agent N-cyano-N-phenyl-para-toluenesulfonamide (NCTS) affords 3-cyanoindoles and 2-cyanopyrroles in good yields with excellent regioselectivity. The substrate scope is broad with respect to indoles and
Daniela Vullo et al.
Bioorganic & medicinal chemistry letters, 15(4), 971-976 (2005-02-03)
The inhibition of a newly cloned human carbonic anhydrase (CA, EC 4.2.1.1), isozyme VII (hCA VII), has been investigated with a series of aromatic and heterocyclic sulfonamides, including some of the clinically used derivatives (acetazolamide, methazolamide, ethoxzolamide, dichlorophenamide, dorzolamide, brinzolamide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service