54127-U
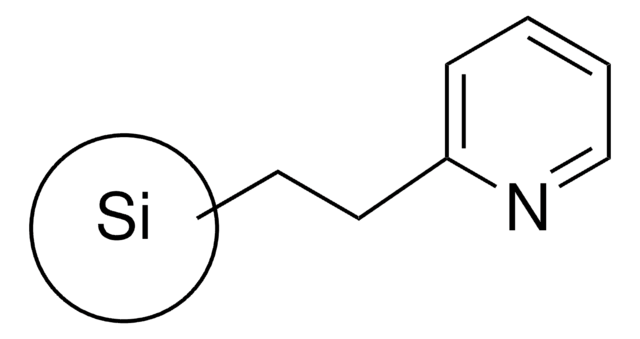
2-(2-Pyridyl)ethyl Silica Gel
bed wt 100 mg, volume 1 mL, pk of 108
About This Item
Recommended Products
composition
bed wt, 100 mg
packaging
pk of 108
technique(s)
solid phase extraction (SPE): suitable
volume
1 mL
matrix active group
WAX phase
application(s)
food and beverages
separation technique
ion exchange
Related Categories
General description
Sample Matrix Compatibility: Organic or aqueous Solutions
- Weak anion exchanger ideal for extracting strong basic compounds that remain charged at all pH levels
- Unlike conventional weak anion exchange SPE phases such as -NH2 (aminopropyl) that have a pKa of 9-10, a pH ≤ 7 is required to protonate or ionize the stationary phase to facilitate analyte retention. Elution is typically done by increasing the pH to 11 resulting in neutralization of the SPE phase.
- 2-(2-pyridyl)-ethyl silica gel has a pKa of ~6. Therefore, analyte elution is feasible at a pH ≥ 7. This characteristic is important for extracting analytes that are not stable (e.g. hydrolyzes) at high pHs typically required for elution when using traditional weak anion exchangers.
- Ideal for extracting acyl-coenzyme A esters from tissue.
- For more information, please see: Minkler, P.E., Kerner, J., Ingalls, S.T., Hoppel, C.L., Novel isolation procedure for short-, medium-, and long-chain acyl-coenzyme A esters from tissue, Analytical Biochemistry 376 (2008) 275–276
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
SPE retention mechanism in this case is based on the electrostatic attraction of charged functional groups of the analyte(s) to oppositely charged functional groups on the sorbent.
Protocols
Retention occurs through polar interaction between the sorbent and analytes. Typical sample matrices that can be employed in normal-phase SPE include hydrocarbon or fatty oils diluted in a solvent like hexane, isooctane, chlorinated solvent, THF, diethyl ether, or ethyl acetate.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service