RAB0630
Human NPPB / Natriuretic Peptides B ELISA Kit
for serum, plasma, cell culture supernatants and urine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41116158
NACRES:
NA.32
Recommended Products
species reactivity
human
packaging
kit of 96 wells (12 strips x 8 wells)
technique(s)
ELISA: suitable
input
sample type urine
sample type cell culture supernatant(s)
sample type plasma
sample type serum
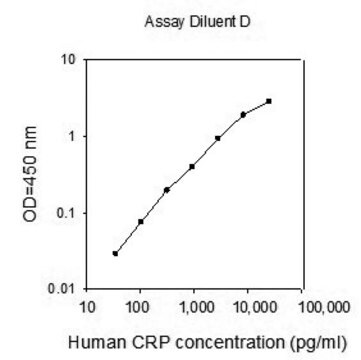
assay range
inter-assay cv: <12%
intra-assay cv: <10%
sensitivity: 14 pg/mL
standard curve range: 15.63-1000 pg/mL
detection method
colorimetric
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... NPPB(4879)
General description
The NPPB ELISA kit provides for the Quantitative measurement of Natriuretic Peptides B in Cell Culture Supernatants, Plasma and Serum.
Application
For research use only. Not for use in diagnostic procedures.
Please refer to the attached General ELISA KIT Procedure (sandwich, competitive & Indirect ELISA)
Please refer to the attached General ELISA KIT Procedure (sandwich, competitive & Indirect ELISA)
Other Notes
A sample Certificate of Analysis is available for this product.
Please type the word sample in the text box provided for lot number.
Please type the word sample in the text box provided for lot number.
Kit Components Also Available Separately
Product No.
Description
SDS
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Met. Corr. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tone B Enger et al.
Journal of cardiothoracic and vascular anesthesia, 31(3), 837-846 (2016-12-28)
To investigate whether a multimarker strategy combining preoperative biomarkers representing distinct pathophysiologic pathways enhances preoperative risk assessment of acute kidney injury after cardiac surgery (CSA-AKI) and increases knowledge of underlying pathogenesis. Prospective, cohort study. Single-center tertiary referral hospital. The study
Sahera Dirajlal-Fargo et al.
AIDS (London, England), 31(14), 1917-1924 (2017-06-08)
Evaluating cardiovascular disease risk in children and youth 13 to 24 years old who are facing a life time exposure to both HIV and antiretroviral therapy is a research priority. This study compares endothelial function measured by peripheral arterial tonometry
Yusuke Ito et al.
FEBS open bio, 8(5), 799-816 (2018-05-11)
5-Hydroxy-3-methylglutaryl-CoA reductase inhibitors (statins) have beneficial effects in patients with heart failure (HF), regardless of serum cholesterol levels. However, their synergic effects with angiotensin II receptor blocker (ARB) remain to be established. We assessed the existence and potential underlying mechanisms
Chunlian Zhong et al.
Oncotarget, 8(33), 54187-54198 (2017-09-15)
Myocarditis is a major cause of sudden, unexpected death in young people. However, it is still one of the most challenging diseases to treat in cardiology. In the present study, we showed that both expression level and activity of PKC-α
Markus Stenemo et al.
European journal of heart failure, 20(1), 55-62 (2017-10-03)
To identify novel risk markers for incident heart failure using proteomic profiling of 80 proteins previously associated with cardiovascular pathology. Proteomic profiling (proximity extension assay) was performed in two community-based prospective cohorts of elderly individuals without heart failure at baseline:
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service