P4399
β-Lactamase from Enterobacter cloacae
Type III, lyophilized powder, 6-18 units/mg protein (using benzylpenicillin)
Synonym(s):
β-Lactamase I, β-Lactamase II, Cephalosporinase, Penicillin amido-β-lactam hydrolase, Penicillinase from Enterobacter cloacae
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
type
Type III
form
lyophilized powder
specific activity
6-18 units/mg protein (using benzylpenicillin)
composition
Protein, ~10%
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Application
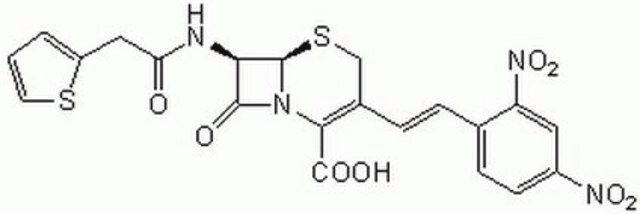
β--lactamase is used to inactivate β-lactam antibiotics by breaking open the β-lactam ring. β--lactamase is used to study antibiotic resistance and resistance suppression. Product P4399 is produced from Enterobacter cloacae.
Biochem/physiol Actions
β--lactamase inactivates β-lactam antibiotics by breaking open the β-lactam ring.
Unit Definition
One unit will hydrolyze 1.0 μmole of benzylpenicillin per min at pH 7.0 at 25 °C. This International Unit (using benzylpenicillin as substrate) is approximately equal to 600 Levy or 75 Pollock units.
Physical form
Lyophilized powder containing sodium phosphate and sodium citrate buffer salts
Preparation Note
Chromatographically purified
Analysis Note
Protein determined by biuret
inhibitor
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P J Johnsen et al.
Genetics, 181(4), 1521-1533 (2009-02-05)
We present a new hypothesis for the selective pressures responsible for maintaining natural competence and transformation. Our hypothesis is based in part on the observation that in Bacillus subtilis, where transformation is widespread, competence is associated with periods of nongrowth
Arnold Louie et al.
Antimicrobial agents and chemotherapy, 56(1), 258-270 (2011-10-26)
New broad-spectrum β-lactamases such as KPC enzymes and CTX-M-15 enzymes threaten to markedly reduce the utility of our armamentarium of β-lactam agents, even our most potent drugs, such as carbapenems. NXL104 is a broad-spectrum non-β-lactam β-lactamase inhibitor. In this evaluation
Ryan M Phelan et al.
Bioorganic & medicinal chemistry letters, 19(4), 1261-1263 (2009-01-27)
An efficient synthesis of a 5-fluorouracil-cephalosporin prodrug is described for use against colorectal and other cancers in antibody and gene-directed therapies. The compound shows stability in aqueous media until specifically activated by beta-lactamase (betaL). The kinetic parameters of the 5-fluorouracil-cephalosporin
Elena De Vecchi et al.
Journal of medical microbiology, 62(Pt 6), 859-863 (2013-03-12)
Urinary tract infections (UTIs) are a common cause of bacteraemia in the elderly and are associated with a high probability of hospitalization. Despite the impact of UTIs on health status and quality of life, a limited number of studies have
A A Alsultan et al.
Journal of medical microbiology, 62(Pt 6), 885-888 (2013-03-23)
Carbapenem-resistant Acinetobacter baumannii is becoming increasingly prevalent in patients with diabetes mellitus in the Middle East. We examined the relationship of these bacteria and their resistance mechanisms to the diabetic disease status of patients in Saudi Arabia. Susceptibilities of 271
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service