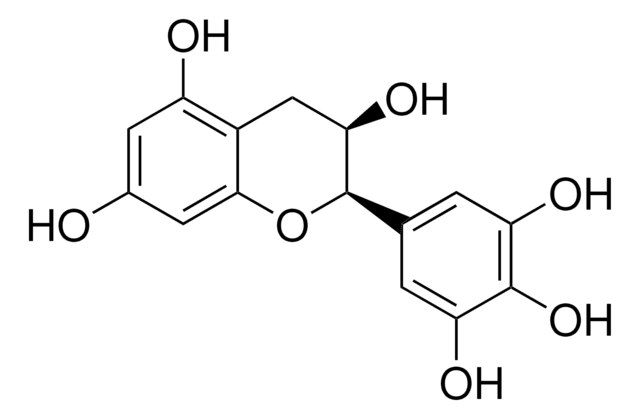
C1251
(+)-Catechin hydrate
≥98% (HPLC), powder
Synonym(s):
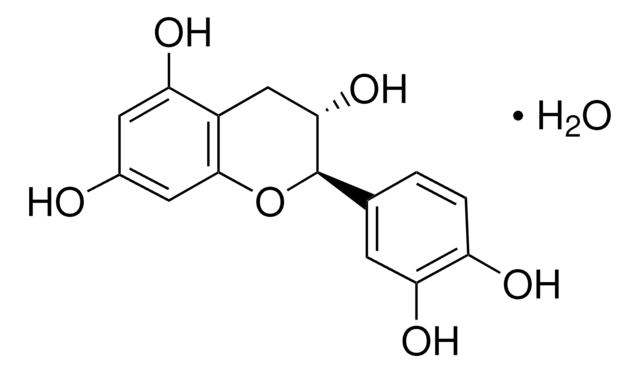
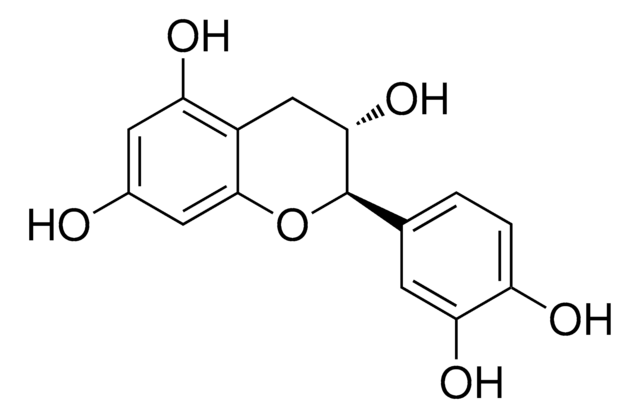
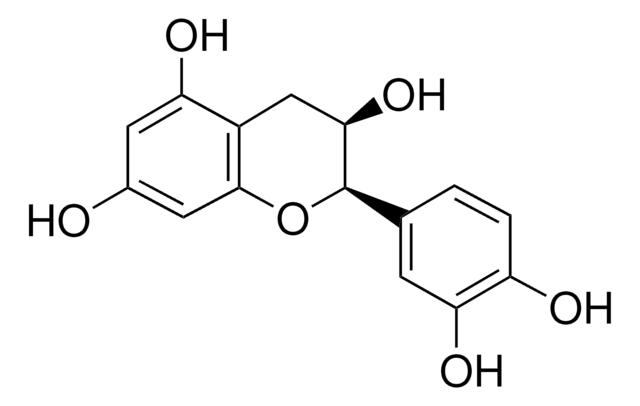
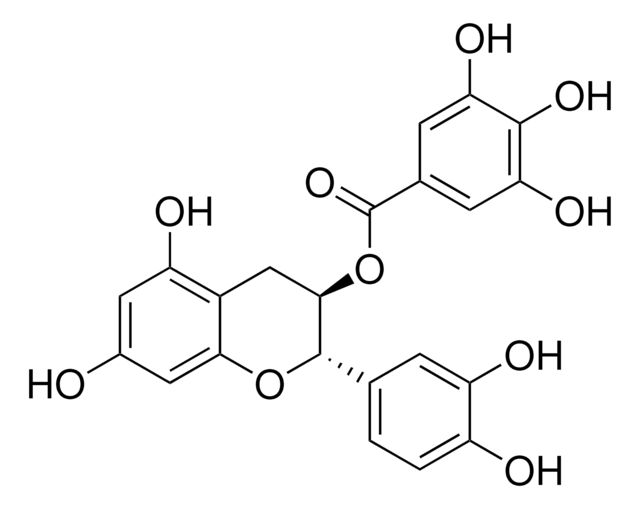
(+)-Cyanidol-3, (2R,3S)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
color
yellow to yellow with tan cast
mp
175-177 °C (anhydrous) (lit.)
solubility
ethanol: 50 mg/mL
storage temp.
2-8°C
SMILES string
[H]O[H].O[C@H]1Cc2c(O)cc(O)cc2O[C@@H]1c3ccc(O)c(O)c3
InChI
1S/C15H14O6.H2O/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7;/h1-5,13,15-20H,6H2;1H2/t13-,15+;/m0./s1
InChI key
OFUMQWOJBVNKLR-NQQJLSKUSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a polyphenol standard in the determination of total polyphenols in the by-products of red wine
- as an additive to study its effects on in vitro methane production and substrate degradation in a triple-fed batch approach
- as a substrate to determine the activity of pure L. plantarum CECT 748T 14 recombinant tannase on catechin
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protocols
Coumaric acid; Quercitrin; Myricetin; Quercetin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service