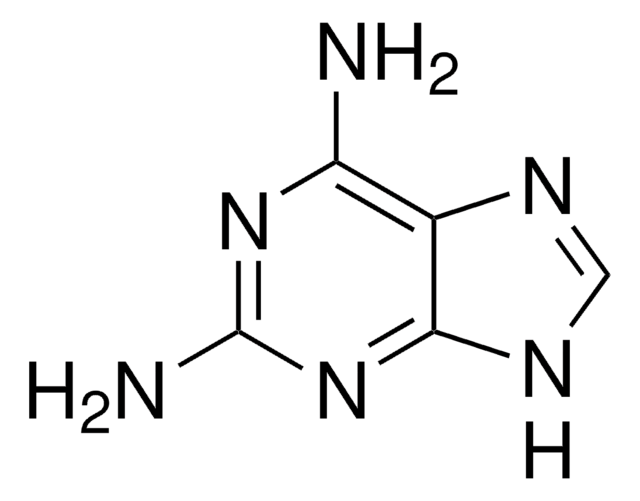
All Photos(5)
About This Item
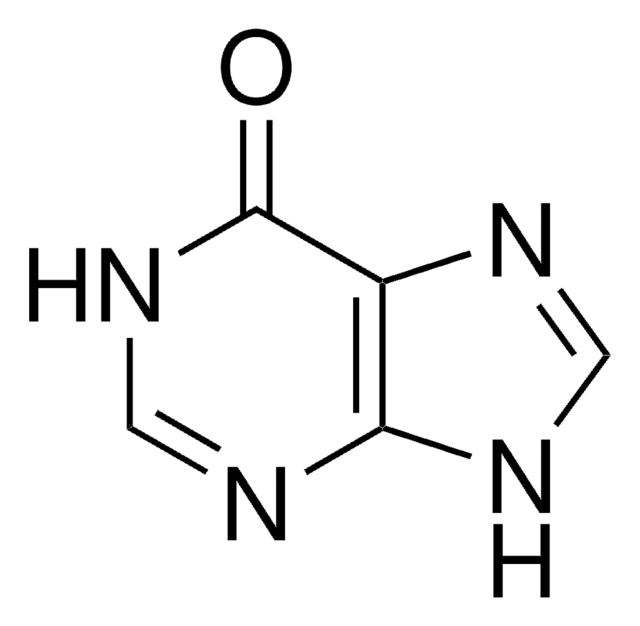
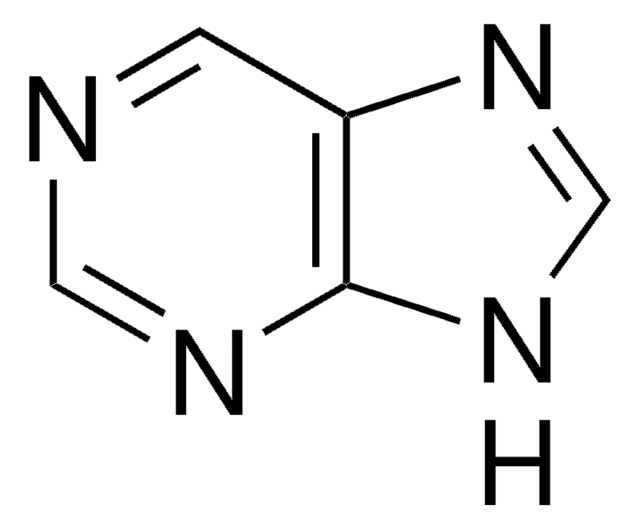
Empirical Formula (Hill Notation):
C5H5N5
CAS Number:
Molecular Weight:
135.13
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic
Assay
≥99%
form
powder
mp
280-282 °C (lit.)
storage temp.
2-8°C
SMILES string
Nc1ncc2[nH]cnc2n1
InChI
1S/C5H5N5/c6-5-7-1-3-4(10-5)9-2-8-3/h1-2H,(H3,6,7,8,9,10)
InChI key
MWBWWFOAEOYUST-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Aminopurine (2-AP) is used to specifically inhibit double-stranded RNA-dependent protein kinase, protein kinase R (PKR).
2-Aminopurine has been used to inhibit eukaryotic initiation factor-2α (eIF2α)-phosphorylation of osteoarthritis (OA) chondrocytes.
Biochem/physiol Actions
2-Aminopurine (2AP) is an analog of guanosine and adenosine, which can base pair with cytosine and thymine. It is used as a fluorescent probe in nucleic acid structure and dynamics. 2-Aminopurine (2-AP) inhibits double-stranded RNA-dependent protein kinase, protein kinase R (PKR).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Endoplasmic reticulum stress induces the expression of COX-2 through activation of eIF2alpha, p38-MAPK and NF-kappaB in advanced glycation end products stimulated human chondrocytes
Rasheed Z and HaqqiTM
Biochimica et Biophysica Acta - Molecular Cell Research, 1823(12), 2179-2189 (2012)
Julien Godet et al.
Nucleic acids research, 41(9), 5036-5048 (2013-03-21)
The HIV-1 nucleocapsid protein (NCp7) is a nucleic acid chaperone required during reverse transcription. During the first strand transfer, NCp7 is thought to destabilize cTAR, the (-)DNA copy of the TAR RNA hairpin, and subsequently direct the TAR/cTAR annealing through
2-Aminopurine.
A Ronen
Mutation research, 75(1), 1-47 (1980-01-01)
2-Aminopurine fluorescence quenching and lifetimes: role of base stacking
Jean JM and Hall KB
Proceedings of the National Academy of Sciences of the USA, 98(1), 37-41 (2001)
Danqing Xin et al.
Brain, behavior, and immunity, 73, 222-234 (2018-05-12)
We previously reported that l-Cysteine, an H2S donor, significantly alleviated brain injury after hypoxia-ischemic (HI) injury in neonatal mice. However, the mechanisms underlying this neuroprotective effect of l-Cysteine against HI insult remain unknown. In the present study, we tested the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service