3023
AptaFluor® SAH Methyltransferase Assay
Synonym(s):
Methyltransferase Activity Assay
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41122100
NACRES:
NA.84
Recommended Products
shipped in
dry ice
storage temp.
−70°C
General description
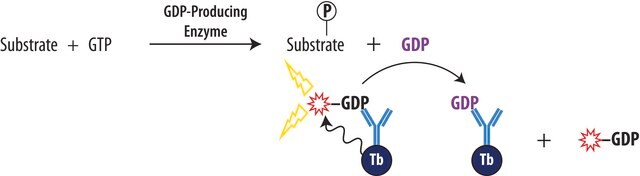
The AptaFluor® SAH Methyltransferase TR-FRET Assay is a universal assay for enzymes that convert S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH), with a far-red, time-resolved Förster-resonance-energy-transfer (TR-FRET) readout. The assay can be used for all enzymes within the histone methyltransferase (HMT) and DNA methyltransferase (DNMT) families. The assay is designed specifically for high-throughput screening (HTS), with a simple mix-and-read format and 2 liquid addition steps. It is run in an endpoint mode: methyltransferase (MT) enzyme reactions are quenched with a universal stop reagent (provided) and then the detection reagents are added.
The assay offers reagent stability and compatibility with commonly used multimode plate readers. The AptaFluor® SAH Methyltransferase TR-FRET Assay provides the following benefits:
View full Transcreener® product list
The assay offers reagent stability and compatibility with commonly used multimode plate readers. The AptaFluor® SAH Methyltransferase TR-FRET Assay provides the following benefits:
- Simple, mix-and-read format ideal for HTS.
- Robust MT detection under initial velocity conditions (=20% conversion of SAM to SAH).
- Accommodates SAM concentrations as low as 100 nM and as high as 5 μM.
- Far-red tracer further minimizes interference from fluorescent compounds and light scattering.
View full Transcreener® product list
Quantity
3023-B = 100 assay, 96-well
3023-1K = 1,000 assay, 384-well
3023-1K = 1,000 assay, 384-well
Physical form
Kit with buffered aqueous solutions
Legal Information
AptaFluor is a registered trademark of BellBrook Labs
Transcreener is a registered trademark of BellBrook Labs
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
L Latini et al.
Cell death & disease, 5, e1404-e1404 (2014-09-05)
Spinal cord injury (SCI) is a devastating condition of CNS that often results in severe functional impairments for which there are no restorative therapies. As in other CNS injuries, in addition to the effects that are related to the primary
Kalyan C Kondapalli et al.
The Journal of biological chemistry, 287(43), 36239-36250 (2012-09-06)
Human NHA2, a newly discovered cation proton antiporter, is implicated in essential hypertension by gene linkage analysis. We show that NHA2 mediates phloretin-sensitive Na(+)-Li(+) counter-transport (SLC) activity, an established marker for hypertension. In contrast to bacteria and fungi where H(+)
Maria Teresa Viscomi et al.
Autophagy, 8(2), 222-235 (2012-01-18)
Autophagy is the evolutionarily conserved degradation and recycling of cellular constituents. In mammals, autophagy is implicated in the pathogenesis of many neurodegenerative diseases. However, its involvement in acute brain damage is unknown. This study addresses the function of autophagy in
Brian G Coon et al.
The Journal of cell biology, 208(7), 975-986 (2015-03-25)
Endothelial responses to fluid shear stress are essential for vascular development and physiology, and determine the formation of atherosclerotic plaques at regions of disturbed flow. Previous work identified VE-cadherin as an essential component, along with PECAM-1 and VEGFR2, of a
Mirja Tamara Prentzell et al.
Cell, 184(3), 655-674 (2021-01-27)
Ras GTPase-activating protein-binding proteins 1 and 2 (G3BP1 and G3BP2, respectively) are widely recognized as core components of stress granules (SGs). We report that G3BPs reside at the cytoplasmic surface of lysosomes. They act in a non-redundant manner to anchor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service