73120
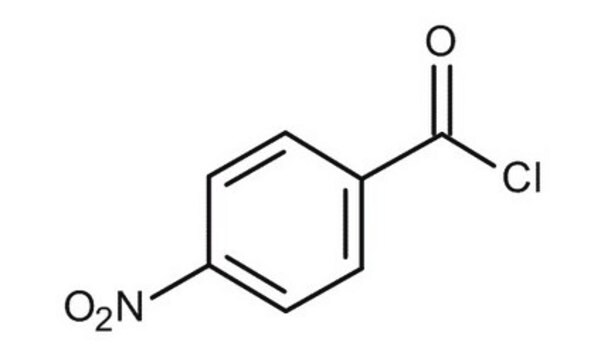
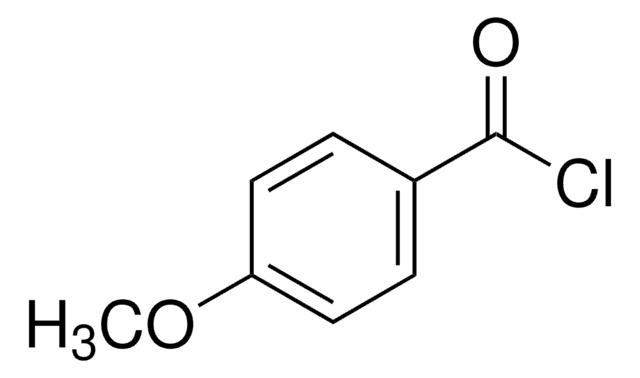
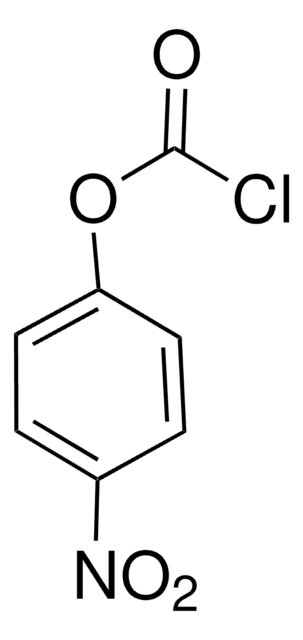
4-Nitrobenzoyl chloride
for HPLC derivatization, LiChropur™, ≥99.0% (GC)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H4COCl
CAS Number:
Molecular Weight:
185.56
Beilstein:
473192
EC Number:
MDL number:
UNSPSC Code:
12000000
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
for HPLC derivatization
Quality Level
Assay
≥99.0% (GC)
form
crystals
quality
LiChropur™
technique(s)
HPLC: suitable
ign. residue
≤0.05% (as SO4)
bp
202-205 °C/105 mmHg (lit.)
mp
71-74 °C (lit.)
71-74 °C
SMILES string
[O-][N+](=O)c1ccc(cc1)C(Cl)=O
InChI
1S/C7H4ClNO3/c8-7(10)5-1-3-6(4-2-5)9(11)12/h1-4H
InChI key
SKDHHIUENRGTHK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Nitrobenzoyl chloride is a derivatization reagent. It reacts with L-glutamic acid undergoing methylation using formaldehyde, synthesizing N-(4-methylaminobenzoyl)glutaminic acid.
Application
4-Nitrobenzoyl chloride was used in separating polyhydric alcohols using HPLC. It was used in snythesizing cellulose benzotates by adding to cellulose/1-allyl-3-methylimidazolium chloride followed by characterization using FT-IF, 1H-NMR and 13C-NMR spectroscopy. It may be used in synthesizing 1-aryloxyacetyl-4-(4-nitrobenzoyl)-thiosemicarbazides under phase transfer catalysis and microwave irradiation with ammonium thiocyanate and aryloxyacetic acid hydrazides.
Other Notes
Derivatization of hydroxy groups for HPLC
Legal Information
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
215.6 °F - closed cup
Flash Point(C)
102 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The synthesis of silica-immobilized 1, 2-naphthoquinone thiosemicarbazone with 3-aminopropyltriethoxy silane and 4-nitrobenzoyl chloride.
Audu, Aramani A.
Reactive Polymers, 10, 3-9 (1989)
Synthesis of cellulose benzoates under homogeneous conditions in an ionic liquid.
Zhang, Jinming, et al.
Cellulose, 16, 299-308 (2009)
Ruben Vardanyan, Victor Hruby
Synthesis of Essential Drugs, 391-391 (2006)
F Nachtmann et al.
Journal of chromatography, 122, 293-303 (1976-07-07)
The separation and quantitative determination of digitalis glycosides by high performance liquid chromatography following derivatization with 4-nitrobenzoylchloride (4-NBC1) is described. The compounds of primary interest were the digitalis glycosides and aglycones of the pharmaceutically important A, B and C series
Journal of Chromatography A, 136, 279-279 (1977)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service