08751
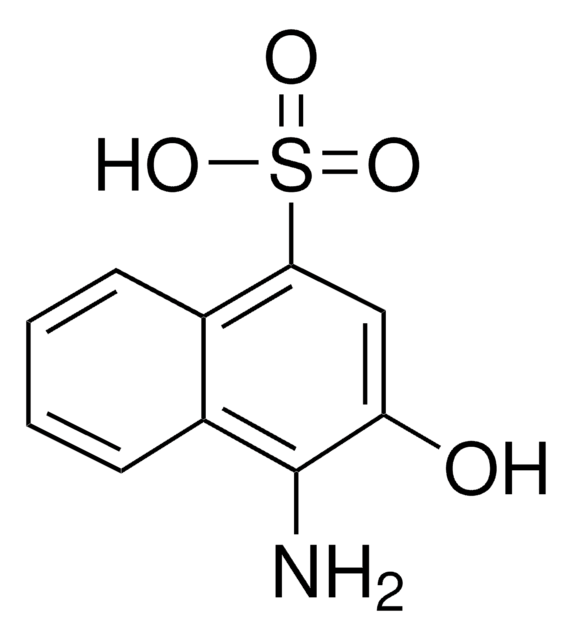
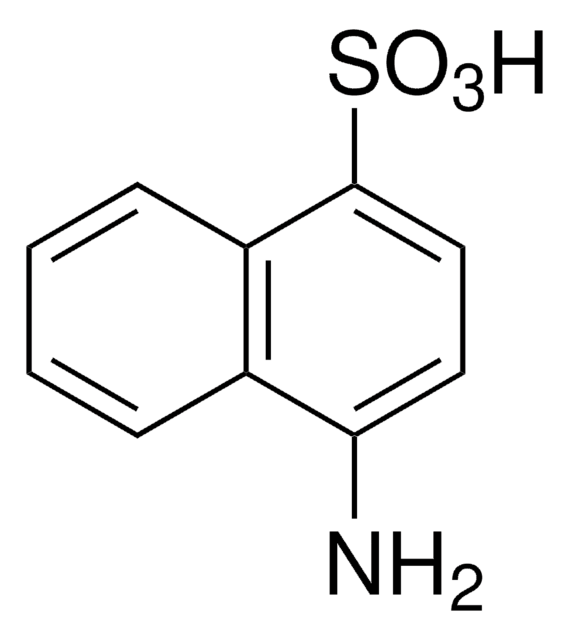
4-Amino-3-hydroxy-1-naphthalenesulfonic acid
for spectrophotometric det. of Si, ≥90.0% (CHN)
Synonym(s):
1-Amino-2-naphthol-4-sulfonic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC10H5(OH)SO3H
CAS Number:
Molecular Weight:
239.25
Beilstein:
2697469
EC Number:
MDL number:
UNSPSC Code:
41116105
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
Assay
≥90.0% (CHN)
form
powder
quality
for spectrophotometric det. of Si
technique(s)
UV/Vis spectroscopy: suitable
mp
290 °C (dec.) (lit.)
SMILES string
Nc1c(O)cc(c2ccccc12)S(O)(=O)=O
InChI
1S/C10H9NO4S/c11-10-7-4-2-1-3-6(7)9(5-8(10)12)16(13,14)15/h1-5,12H,11H2,(H,13,14,15)
InChI key
RXCMFQDTWCCLBL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Amino-3-hydroxy-1-naphthalenesulfonic acid is an aromatic amino-sulfonic acid.
Application
4-Amino-3-hydroxy-1-naphthalenesulfonic acid has been used to study separation of aromatic sulfonated compounds using Ion-interaction high-performance liquid chromatography and micellar electrokinetic capillary chromatography techniques. It has also been used as a starting material which when reacted with palmitoyl chloride yields the following derivatives:
- 2′-Pentadecylnaphth[3,4-d]oxazole-1-sulfonic acid
- 4-Amino-3-(palmitoyloxy)-1-naphthalenesufonic acid
- 4-(Palmitoylamino)-3-hydroxy-1-naphthalenesulfonic acid
- 4-(Palmitoylamino)-3-(palmitoyloxy)-1-naphthalenesulfonic acid
Other Notes
Reagent for the spectrophotometric det. of silicon
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ion-interaction high-performance liquid chromatography and micellar electrokinetic capillary chromatography: two complementary techniques for the separation of aromatic sulfonated compounds.
Angelino, S., et al.
Journal of Chromatography A, 845.1, 257-271 (1999)
Synthesis of naphthalenesulfonic acid small molecules as selective inhibitors of the DNA polymerase and ribonuclease H activities of HIV-1 reverse transcriptase.
Mohan P
Journal of Medicinal Chemistry, 37(16), 2513-2519 (1994)
F.A. Sorrentino et al.
Microchemical Journal, Devoted to the Application of Microtechniques in All Branches of Science, 15, 441-441 (1970)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service